Human Vitronectin N-GST (full-length)
This recombinant full-length VN protein is expressed as a GST-tagged fusion protein in E. coli, liberated from inclusion bodies by urea treatment, and purified over heparin–agarose.
Vitronectin (VN) is a 75-kDa adhesive glycoprotein that also plays a role in regulating several proteolytic enzyme cascades including complement, thrombosis, and fibrinolysis. Several distinct, biologically active conformations of VN are known to exist. In the blood, VN circulates in a monomeric form. High molecular mass oligomers of VN are found within the extracellular matrix (ECM)1 and in platelet releasate. The interaction of vitronectin with thrombin–antithrombin III complexes, plasminogen activator inhibitor, or with chemical or thermal denaturants results in conformational changes that lead to VN multimerization. These conformational changes may be important for VN activity as the multimeric form of VN preferentially binds to v 3 and IIb 3 integrins, urokinase receptors, plasminogen activator inhibitor, and glycosaminoglycans. The interaction of multimeric VN with these ligands affects a number of functions including cell adhesion and spreading, cell migration, inflammation, and hemostasis.
From the laboratory of Denise C. Hocking, PhD, University of Rochester Medical Center.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
This recombinant full-length VN protein is expressed as a GST-tagged fusion protein in E. coli, liberated from inclusion bodies by urea treatment, and purified over heparin–agarose.
Vitronectin (VN) is a 75-kDa adhesive glycoprotein that also plays a role in regulating several proteolytic enzyme cascades including complement, thrombosis, and fibrinolysis. Several distinct, biologically active conformations of VN are known to exist. In the blood, VN circulates in a monomeric form. High molecular mass oligomers of VN are found within the extracellular matrix (ECM)1 and in platelet releasate. The interaction of vitronectin with thrombin–antithrombin III complexes, plasminogen activator inhibitor, or with chemical or thermal denaturants results in conformational changes that lead to VN multimerization. These conformational changes may be important for VN activity as the multimeric form of VN preferentially binds to v 3 and IIb 3 integrins, urokinase receptors, plasminogen activator inhibitor, and glycosaminoglycans. The interaction of multimeric VN with these ligands affects a number of functions including cell adhesion and spreading, cell migration, inflammation, and hemostasis.
From the laboratory of Denise C. Hocking, PhD, University of Rochester Medical Center.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
| Product Type: | Protein |
| Name: | Recombinant Human Vitronectin (full-length); amino acids D1-L459 |
| Accession ID: | P04004 |
| Source: | Human protein expressed in E. coli DH5 alpha carrying the cloned gene in pGEX-2T |
| Molecular Weight: | 78597.5 Da |
| Amino Acid Sequence: | DQESCKGRCTEGFNVDKKCQCDELCSYYQSCCTDYTAECKPQVTRGDVFTMPEDEYTVYDDGEEKNNATVHEQVGGPSLTSDLQAQSKGNPEQTPVLKPEEEAPAPEVGASKPEGIDSRPETLHPGRPQPPAEEELCSGKPFDAFTDLKNGSLFAFRGQYCYELDEKAVRPGYPKLIRDVWGIEGPIDAAFTRINCQGKTYLFKGNQYWRFEDGVLDPDYPRNISDGFDGIPDNVDAALALPAHSYSGRERVYFFKGKQYWEYQFQHQPSQEECEGSSLSAVFEHFAMMQRDSWEDIFELLFWGRTSAGTRQPQFISRDWHGVPGQVDAAMAGRIYISGMAPRPSLTKKQRFRHRNRKGYRSQRGHSRGRNQNSRRPSRAMWLSLFSSEESNLGANNYDDYRMDWLVPATCEPIQSVFFFSGDKYYRVNLRTRRVDTVDPPYPRSIAHYWLGCPAPGHL |
| Fusion Tag(s): | GST, N-terminal |
| Purity: | > 80% by SDS-PAGE |
| Buffer: | Solution in PBS |
| Concentration: | 2.2mg/mL |
| Storage: | Store at -80C |
| Shipped: | Dry ice |
| Fig. 1. SDSPAGE analysis of GST/VN. DH5alpha bacteria were transformed with a pGEX2T vector containing the cDNA of human VN. Samples were obtained at various steps in the purification process, electrophoresed on a 10% gel, and visualized by staining with Coomassie blue. In lane 1, bacteria were grown in LB media overnight, diluted 1:10 in the morning, and then grown for an additional 2 h before a gel sample (25 ul) was obtained. IPTG was then added to induce protein production. After 4 h (lane 2), bacteria were pelleted by centrifugation, resuspended in 2M urea buVer (lane 3), and centrifuged. The resulting supernatant is shown in lane 4. To solubilize the inclusion bodies, the pellet was resuspended in 8M urea buffer andcentrifuged. The supernatant is shown in lane 5. Five micrograms of purified, reduced (lane 6) and non-reduced (lane 7) GST/VN is shown. Samples in lanes 15 were prepared with reducing sample buffer. The positions of the molecular mass markers are indicated. Bracket indicates positions of GST/VN. Adapted from: Wojciechowski, K., Chang, C.H., and Hocking, D.C. (2004) Expression, production, and characterization of full-length vitronectin in Escherichia coli. Protein Express. Purif. 36:131-138. | 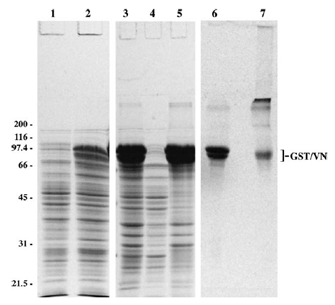 |
- Wojciechowski, K., Chang, C.H., and Hocking, D.C. (2004) Expression, production, and characterization of full-length vitronectin in Escherichia coli. Protein Express. Purif. 36:131-138.
If you publish research with this product, please let us know so we can cite your paper.