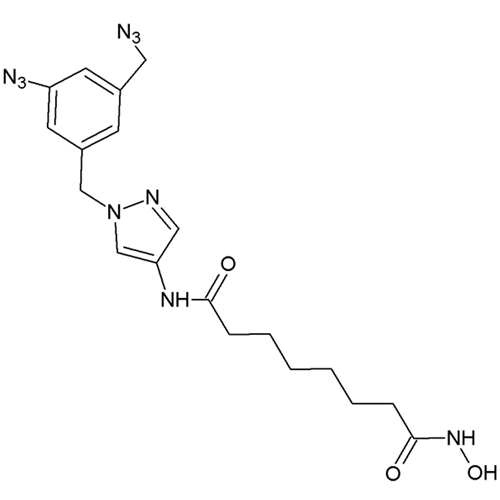
HDAC8-Selective Photoreactive Inhibitor; HD-55
HD-55 is a pyrazole-based compound which acts as a photoreactive histone deacetylase (HDAC) inhibitor suitable for photoaffinity labeling experiments. This allows for the identification of drug target proteins of biologically active compounds and mapping their binding sites.
HD-55 is one of the most active histone deacetylase HDAC8 inhibitors reported to date, more selective than even Trichostatin A (TSA) or suberoylanilide hydroxamic acid (SAHA). This is presumably due to the flexibility of the pyrazole moiety to allow for occupancy of the second HDAC8 binding site. Additionally, it is highly specific for HDAC8, showing an 8-fold selectivity verses HDAC3. Cell-based studies show HD-55 is able to freely enter the cell nucleus and trigger mechanisms known to respond to HDAC inhibition such as accumulation of acetylated H4, activation of caspase 3, and cell death of highly proliferative human cancer cell lines at low micromolar concentrations. Additionally, unlike SAHA, HD-55, does not exert cytotoxic effect in neuronal-like cells and even protected these cells from chemically induced apoptosis.
From the laboratory of Pavel A. Petukhov, PhD, University of Illinois at Chicago.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
HD-55 is a pyrazole-based compound which acts as a photoreactive histone deacetylase (HDAC) inhibitor suitable for photoaffinity labeling experiments. This allows for the identification of drug target proteins of biologically active compounds and mapping their binding sites.
HD-55 is one of the most active histone deacetylase HDAC8 inhibitors reported to date, more selective than even Trichostatin A (TSA) or suberoylanilide hydroxamic acid (SAHA). This is presumably due to the flexibility of the pyrazole moiety to allow for occupancy of the second HDAC8 binding site. Additionally, it is highly specific for HDAC8, showing an 8-fold selectivity verses HDAC3. Cell-based studies show HD-55 is able to freely enter the cell nucleus and trigger mechanisms known to respond to HDAC inhibition such as accumulation of acetylated H4, activation of caspase 3, and cell death of highly proliferative human cancer cell lines at low micromolar concentrations. Additionally, unlike SAHA, HD-55, does not exert cytotoxic effect in neuronal-like cells and even protected these cells from chemically induced apoptosis.
From the laboratory of Pavel A. Petukhov, PhD, University of Illinois at Chicago.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
| Product Type: | Small Molecule |
| Name: | HD-55 |
| Chemical Formula: | C19H24N10O3 |
| Molecular Weight: | 440.5 |
| Format: | solid |
| Purity: | >95%, LCMS |
| Stability: | >6 months when stored at -20C |
| Comments: | Photoreactive group: arylazide; Activation wavelength: 280nm; Activation period: 3min |
| Storage: | Store at -20C |
| Shipped: | Cold packs |
| Compound | IC50 ± SD (nM) | HDAC8/HDAC3 | |
| HDAC3 | HDAC8 | ||
| SAHA | 27 ± 1.0 | 440 ± 21 | 16.3 |
| HD-55 | 128 ± 9.8 | 17 ± 3.0 | 0.13 |
Adapted from Table 2 & 3; Neelarapu, R., et al. Journal of Medicinal Chemistry. 54: 4350 - 4364. 2011.
- Neelarapu, R., et al. Design, Synthesis, Docking, and Biological Evaluation of Novel Diazide-Containing Isoxazole- and Pyrazole-Based Histone Deacetylase Probes. Journal of Medicinal Chemistry. 54: 4350 - 4364. 2011.
If you publish research with this product, please let us know so we can cite your paper.