Anti-Prion Protein [PrioV] Antibody
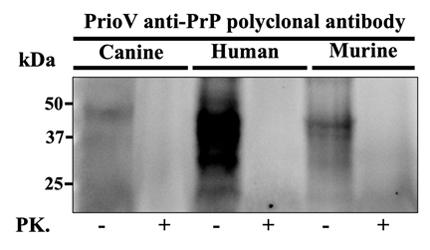
This camelid (alpaca) IgG polyclonal antibody was generated against Prion protein (PrP) derived from murine RML-affected brain tissue and recognizes PrP from human, mouse, dog, sheep, and cow.
Highlights
- Shown to recognize PrP from human, mouse, dog, sheep, and cow
- Recommended for ELISA, Western blot, IHC, and IF applications
Prions are misfolded proteins with the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. PRNP is the human gene encoding for the major prion protein PrP, also known as CD230. The normal form of the protein is called PrPC, while the infectious form is called PrPSc - the C refers to 'cellular' PrP, while the Sc refers to 'scrapie', the prototypic prion disease, occurring in sheep.
From the laboratory of Mourad Tayebi, PhD, Western Sydney University.
This camelid (alpaca) IgG polyclonal antibody was generated against Prion protein (PrP) derived from murine RML-affected brain tissue and recognizes PrP from human, mouse, dog, sheep, and cow.
Highlights
- Shown to recognize PrP from human, mouse, dog, sheep, and cow
- Recommended for ELISA, Western blot, IHC, and IF applications
Prions are misfolded proteins with the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. PRNP is the human gene encoding for the major prion protein PrP, also known as CD230. The normal form of the protein is called PrPC, while the infectious form is called PrPSc - the C refers to 'cellular' PrP, while the Sc refers to 'scrapie', the prototypic prion disease, occurring in sheep.
From the laboratory of Mourad Tayebi, PhD, Western Sydney University.
| Product Type: | Antibody |
| Antigen: | PrPSc derived from murine RML-affected brain tissue |
| Accession ID: | P04156, F7VJQ1.1 |
| Molecular Weight: | 30kDa |
| Isotype: | IgG |
| Clonality: | Polyclonal |
| Clone Name: | PrioV |
| Reactivity: | human, mouse, dog, sheep, cow |
| Immunogen: | Brain homogenate derived from murine RML-affected brain tissue |
| Species Immunized: | Alpaca |
| Purification Method: | Antibody purification was performed using the BIORAD NGC 10 Medium-Pressure Chromatography Systems |
| Method Used to Determine Concentration: | BCA |
| Buffer: | PBS |
| Tested Applications: | ELISA (1ug/mL), Western Blot (1ug/mL), IHC (1:500), IF (1:500) |
| Concentration: | 1mg/mL |
| Amount: | 100ug |
| Comments: | Brain homogenate adsorbed to immunomagnetic beads and emulsified in CFA and final booster in IFA.; References included refer to mAbs produced from the same animals the pAbs were derived from. |
| Storage: | -20C |
| Shipped: | Cold Packs |
- Jones DR, Taylor WA, Bate C, David M, Tayebi M. A camelid anti-PrP antibody abrogates PrP replication in prion-permissive neuroblastoma cell lines. PLoS One. 2010;5(3):e9804. Published 2010 Mar 22. doi:10.1371/journal.pone.0009804
- David MA, Jones DR, Tayebi M. Potential candidate camelid antibodies for the treatment of protein-misfolding diseases. J Neuroimmunol. 2014 Jul 15;272(1-2):76-85. doi: 10.1016/j.jneuroim.2014.05.001. Epub 2014 May 10. PMID: 24864011.
If you publish research with this product, please let us know so we can cite your paper.

![Anti-Prion Protein [PrioV] Antibody Anti-Prion Protein [PrioV] Antibody](https://www.kerafast.com/MediaStorage/Product/Images/Medium/19_20210622134721189.jpg)
