MetalloBuffer BufferTM Kit pZn 13-9
MetalloBufferTM Buffer Kit pZn 13-9 is a set of 2x 10mM HEPES 10 mM NTA 125 mM NaCl pH 7.5 buffers that when mixed together as directed provide fixed, low free zinc concentrations between 0.1 pM (pZn = 13) and 1.0 nM (pZn = 9) at 25C.
To calibrate the response of sensors such as fluorescent chemosensors against low concentrations of free (mobile, exchangeable) zinc ion near physiological pH is difficult, because of contaminating metal ions, the propensity of zinc ions to bind to ligands or glass surfaces, and the tendency of zinc ions to hydrolyse and precipitate near neutral pH. MetalloBuffersTM minimize these issues by effectively buffering the free zinc ion levels in a manner analogous to pH buffers. MetalloBufferTM pZn levels are corrected for ionic strength and temperature effects.
Read plog post from Dr. Richard Thompson at Pokegama Technologies, Improving Zinc Ion Quantitation with Unique Biosensor Proteins and Buffers.
MetalloBufferTM Buffer Kit pZn 13-9 is a set of 2x 10mM HEPES 10 mM NTA 125 mM NaCl pH 7.5 buffers that when mixed together as directed provide fixed, low free zinc concentrations between 0.1 pM (pZn = 13) and 1.0 nM (pZn = 9) at 25C.
To calibrate the response of sensors such as fluorescent chemosensors against low concentrations of free (mobile, exchangeable) zinc ion near physiological pH is difficult, because of contaminating metal ions, the propensity of zinc ions to bind to ligands or glass surfaces, and the tendency of zinc ions to hydrolyse and precipitate near neutral pH. MetalloBuffersTM minimize these issues by effectively buffering the free zinc ion levels in a manner analogous to pH buffers. MetalloBufferTM pZn levels are corrected for ionic strength and temperature effects.
Read plog post from Dr. Richard Thompson at Pokegama Technologies, Improving Zinc Ion Quantitation with Unique Biosensor Proteins and Buffers.
| Product Type: | Buffer or Chemical |
| Name: | MetalloBufferTM Buffer Kit pZn 13-9 |
| Product MPN: | M-0005 |
| Format: | Set of bottles containing differing total zinc concentrations |
| Tested Applications: | Calibration of fluorescent zinc sensors |
| Solubility: | Aqeuous solutions |
| Storage: | Room Temperature |
| Shipped: | Room Temperature |
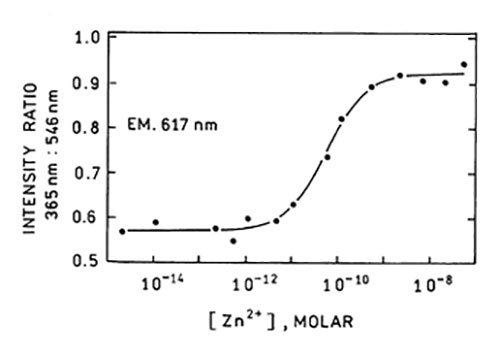
Shown is a ratiometric calibration curve of a fluorescent-labeled apocarbonic anhydrase II with excitation at 365 and 546 nm, and emission at 617 nm.
- R.A. Bozym, T. Hurst, N. Westerberg, A.V. Stoddard, C.A. Fierke, C.J. Frederickson, R.B. Thompson, "Determination of zinc using carbonic anhydrase-based fluorescence biosensors" in Methods in Enzymology: Fluorescence Spectroscopy Vol. 450 (L. Brand and M.L. Johnson, editors) New York: Elsevier, pp 279-301 (2008). PMID 19152866.
- W. Stumm and J.J. Morgan, Aquatic Chemistry, 3rd Edition (Wiley-Interscience) 1996.
If you publish research with this product, please let us know so we can cite your paper.


