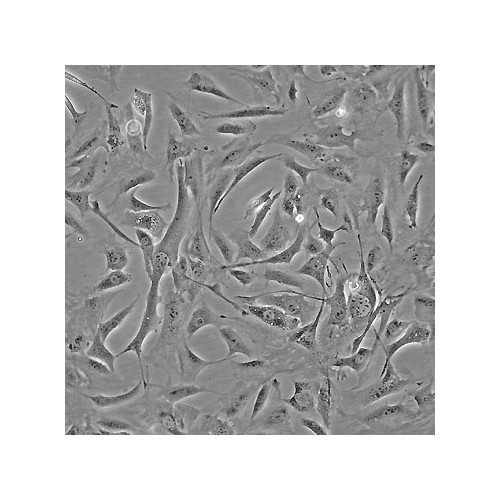
3T3 Preadipose Cell Lines
3T3 L1 and 3T3 F442A are clonal sublines isolated from 3T3 mouse embryonic fibroblasts, and can be differentiated to adipocytes. These cell lines are commonly used a model to study fat metabolism.
Highlights:
- After differentiation, these cells accumulate large amounts of triglyceride fat
- Triglyceride fat accumulation can be reduced by lipolytic agents
- Express insulin receptor
- 3T3 L1 accumulates triglyceride fat to a lesser extent than 3T3 F442A
Adipose tissue is crucial in energy storage and metabolic homeostasis. An increase in adipose tissue results either from an enlargement of mature adipocytes or from the differentiation of adipocyte precursor cells (preadipocytes) into new mature adipocytes. These preadipocytes are already present in adipose tissues. They can proliferate throughout adult life and replace the cells that have differentiated into mature adipocytes. Since this differentiation process is controlled by a variety of growth factors and hormones, preadipocytes are suitable for the investigation of the physiological mechanisms controlling proliferation, differentiation, and function of adipose tissue.
From the laboratory of Howard Green, MD, Harvard University.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
3T3 L1 and 3T3 F442A are clonal sublines isolated from 3T3 mouse embryonic fibroblasts, and can be differentiated to adipocytes. These cell lines are commonly used a model to study fat metabolism.
Highlights:
- After differentiation, these cells accumulate large amounts of triglyceride fat
- Triglyceride fat accumulation can be reduced by lipolytic agents
- Express insulin receptor
- 3T3 L1 accumulates triglyceride fat to a lesser extent than 3T3 F442A
Adipose tissue is crucial in energy storage and metabolic homeostasis. An increase in adipose tissue results either from an enlargement of mature adipocytes or from the differentiation of adipocyte precursor cells (preadipocytes) into new mature adipocytes. These preadipocytes are already present in adipose tissues. They can proliferate throughout adult life and replace the cells that have differentiated into mature adipocytes. Since this differentiation process is controlled by a variety of growth factors and hormones, preadipocytes are suitable for the investigation of the physiological mechanisms controlling proliferation, differentiation, and function of adipose tissue.
From the laboratory of Howard Green, MD, Harvard University.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
| Catalog Number | Product | DataSheet | Size | AVAILABILITY | Price | Qty |
|---|
| Product Type: | Cell Line |
| Name: | 3T3 L1 & 3T3 F442A |
| Cell Type: | Embryonic mouse fibroblast preadipose cell line |
| Accession ID: | L1, CVCL_0123; F442A, CVCL_0122 |
| Organism: | Mouse |
| Morphology: | Fibroblastic, can be differentiated to adipocytes |
| Source: | Mouse embryo |
| Biosafety Level: | BL1 |
| Growth Conditions: | Cells grow in 10% CO2 at 37C in a humidified incubator. Media: DMEM (high glucose, no sodium pyruvate and no HEPES) supplemented with 10% bovine calf serum; penn/strep and 2mM L-Glutamine may be added. DO NOT use fetal bovine serum as this will cause increased background differentiation. DO NOT use fetal bovine serum. Growth in fetal bovine serum affects the growth potential of these cells. Bovine calf serum should be iron supplemented and NOT gamma irradiated or heat inactivated (Recommended serum: GE Healthcare Iron-Supplemented BVN CLF 500ml; Cat.SH30072.03). |
| Subculturing: | Subculture the cells once they reach 60-80% confluency It is very important not to let the cells get confluent or they will begin to spontaneously differentiate. Split the cells at a density of ~ 3.3 x 103cells/cm2. Feed every 2 to 3 days with complete medium. |
| Cryopreservation: | Complete media with 10% sterile DMSO |
| Storage: | Liquid nitrogen |
| Shipped: | Dry ice |
When thawing these cells, do not centrifuge, as this can lead to a poor recovery rate. The providing laboratory recommends:
- Thaw vial using 37C water bath
- Bring vial into hood and rinse with alcohol
- Using a pasteur pipet, transfer contents into a centrifuge tube
- Add the desired amount of medium dropwise, gently swirling to mix after each drop DO NOT add the medium too quickly or the cells will burst. NOTE: The order is very important: medium should be added, dropwise, to the cells. Do not prepare a tube of medium and add the entire vial of cells to it or you will end up losing a lot of cells.
To differentiate 3T3-F442A cells to adipocytes see: Djian, P., Phillips, M., and Green, H. (1985). The activation of specific gene transcription in the adipose conversion of 3T3 cells. J Cell Physiol 124, 554-6. The 3T3-F442A line should be 80-90% differentiated after 2 weeks in FBS and insulin. 3T3-L1 differentiation is less extensive than that of 3T3-F442A under the same conditions.
- Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974 Oct;3(2):127-33.
- Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19-27.
- Green H, Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105-13.
- Djian P, Phillips M, Green H. The activation of specific gene transcription in the adipose conversion of 3T3 cells. J Cell Physiol. 1985 Sep;124(3):554-6.
- Doğan A, Demirci S, Kıratlı B, Şahin F. Cytoglobin: a potential marker for adipogenic differentiation in preadipocytes in vitro. Cytotechnology. 2017 Feb;69(1):157-165. View Article
- Wu B, Sun X, Gupta HB, Yuan B, Li J, Ge F, Chiang HC, Zhang X, Zhang C, Zhang D, Yang J, Hu Y, Curiel TJ, Li R. Adipose PD-L1 Modulates PD-1/PD-L1 Checkpoint Blockade Immunotherapy Efficacy in Breast Cancer. Oncoimmunology. 2018 Aug 23;7(11):e1500107. View Article
- Pydi SP, Jain S, Tung W, Cui Y, Zhu L, Sakamoto W, Jain S, Abel BS, Skarulis MC, Liu J, Huynh T, Pacak K, Caron MG, Gavrilova O, Finkel T, Wess J. Adipocyte β-arrestin-2 is essential for maintaining whole body glucose and energy homeostasis. Nat Commun. 2019 Jul 3;10(1):2936. View Article
If you publish research with this product, please let us know so we can cite your paper.