Hyperstable Hsp60 (Pf Cpn) Protein
This hyperstable chaperonin Hsp60 (Pf Cpn) from Pyrococcus furiosus was recombinantly produced in E. coli and posesses non-native protein salvage activity including ability to deconstruct amyloid fibrils (prions).
Pyrococcus furiosus is an hyperthermophilic species of Archaea. It is notable for having an optimum growth temperature of 100C. Hyperstable Hsp60 (Pf Cpn) Protein acts as a chaperone to enhance protein
folding and increase enzyme or microbial resistance to heat. Dr. Robb found that the thermostability
of Taq polymerase was significantly improved by combinations of P. furiosus chaperones, showing ongoing protein folding activity at elevated temperatures and during thermal cycling. These properties may be exploited to enhance the durability and cost effectiveness of high temperature biocatalysts.
From the laboratory of Frank T. Robb, PhD, University of Maryland, Baltimore, Institute of Marine and Environmental Technology (IMET).
This hyperstable chaperonin Hsp60 (Pf Cpn) from Pyrococcus furiosus was recombinantly produced in E. coli and posesses non-native protein salvage activity including ability to deconstruct amyloid fibrils (prions).
Pyrococcus furiosus is an hyperthermophilic species of Archaea. It is notable for having an optimum growth temperature of 100C. Hyperstable Hsp60 (Pf Cpn) Protein acts as a chaperone to enhance protein folding and increase enzyme or microbial resistance to heat. Dr. Robb found that the thermostability of Taq polymerase was significantly improved by combinations of P. furiosus chaperones, showing ongoing protein folding activity at elevated temperatures and during thermal cycling. These properties may be exploited to enhance the durability and cost effectiveness of high temperature biocatalysts.
From the laboratory of Frank T. Robb, PhD, University of Maryland, Baltimore, Institute of Marine and Environmental Technology (IMET).
| Product Type: | Protein |
| Name: | HSP60, Pf Cpn |
| Accession ID: | P10809, PFC_08710 (JGI) |
| Source: | E. coli BL21 |
| Molecular Weight: | 60 kDa |
| Amino Acid Sequence: | MAQLAGQPILILPEGTQRYVGRDAQRMNILAARIVAETIRTTLGPKGMDK MLVDSLGDIVITNDGATILDEMDIQHPAAKMMVEVAKTQDKEAGDGTTTA VVIAGELLRKAEELLDQNIHPSIIIKGYTLAAQKAQEILENIAKEVKPDD EEILLKAAMTSITGKAAEEEREYLAKLAVEAVKLVAEKEDGKYKVDIDNI KLEKKEGGSVRDTQLIRGVVIDKEVVHPGMPKRVEKAKIALINDALEVKE TETDAEIRITSPEQLQAFLEQEERMLREMVEKIKEVGANVVFVQKGIDDL AQHYLAKYGIMAVRRVKKSDMEKLAKATGAKIVTNIRDLTPEDLGYAELV EERKVAGESMIFVEGCQNPKAVTILIRGGTEHVVDEVERALEDAIKVVKD ILEDGKILAGGGAPEIELAIRLDEYAKEVGGKEQLAIEAFAEALKVIPRT LAENAGLDPIETLVKVIAAHKEKGPTIGVDVYEGEPADMLERGVIEPLRV KKQAIKSASEAAIMILRIDDVIAASKLEKEKEKEGEKGGGSEDFSSDLD |
| Purity: | 100 |
| Buffer: | HEPES 50 mM, pH7.5 |
| Concentration: | 1 mg/mL |
| Activity: | ug/min/mg ATPase |
| Suggested Amount per Experiment: | 50 ng |
| Comments: | Hyperstable. Hexadecamer, 1mDa complex |
| Storage: | -80C |
| Shipped: | Dry Ice |
Purity Analysis of Recombinant Pf Cpn.
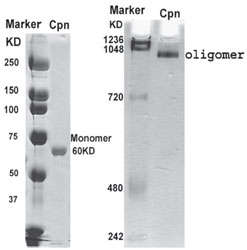
The recombinant Pf Cpn was analyzed by SDS–PAGE (5 ug) and 6% Native gel (20 ug). The native gel markers from top to bottom are IgM Hexamer, 1236 kDa; IgM Pentamer, 1048 kDa; Apoferritin band 1, 720 kDa; Aproferritin band 2, 480 kDa; and B-phycoerythrin, 242 kDa.
Adapted from: Luo H, et al. (2009) Arch Biochem Biophys. 486:12-18.
- Sun, Y., N. Makarava, C-I. Lee, P. Laksanalamai, F. T. Robb and I. V. Baskakov (2008) Conformational stability of PrP amyloid fibrils controls their smallest possible fragment size. J. Molecular Biology 376(4):1155 - 1167.
- Luo H, Laksanalamai P, Robb FT. (2009) An exceptionally stable Group II chaperonin from the hyperthermophile Pyrococcus furiosus. Arch Biochem Biophys. 486:12-18.
- Kurouski D, Luo H, Sereda V, Robb FT, Lednev IK (2013) Deconstruction of stable cross-Beta fibrillar structures into toxic and nontoxic products using a mutated archaeal chaperonin. ACS Chem Biol. 2013 Sep 20;8(9):2095-101. doi: 10.1021/cb400238
If you publish research with this product, please let us know so we can cite your paper.