Human Norrin Protein
Recombinant mature form (amino acids 25-133) of human norrin expressed in HEK-293T cells and purified utilizing affinity and size exclusion chromatography.
Highlights:
- Highly Pure - >99% (SDS-PAGE)
- Biologically Active - Shown to active the canonical Wnt/β-catenin pathway in luciferase reporter assay
Norrin (Norrie Disease Protein) is a cystine-knot like growth factor that can activate the canonical Wnt signaling pathway through FZD4 and LRP5/6 coreceptor. This group or ‘complex’ also includes molecules called glycosaminoglycans. Norrin plays a central role in retinal vascularization by acting as a ligand for FZD4 that signals via stabilizing beta-catenin (CTNNB1) and activating LEF/TCF-mediated transcriptional programs. The protein also acts in concert with TSPAN12 to activate FZD4 independently of the Wnt-dependent activation of FZD4, suggesting the existence of a Wnt-independent signaling that also promote accumulation the beta-catenin (CTNNB1). Norrin may be involved in a pathway that regulates neural cell differentiation and proliferation. Wnt signalling regulates multiple processes including angiogenesis, inflammation, and tumorigenesis. In humans, mutations in the gene that encodes Norrin can cause a disease in which blood vessels in the eye fail to form correctly, which can result in blindness. However, it is not clear how Norrin activates Wnt signalling.
From a laboratory at Cancer Research Technology.
Recombinant mature form (amino acids 25-133) of human norrin expressed in HEK-293T cells and purified utilizing affinity and size exclusion chromatography.
Highlights:
- Highly Pure - >99% (SDS-PAGE)
- Biologically Active - Shown to active the canonical Wnt/β-catenin pathway in luciferase reporter assay
Norrin (Norrie Disease Protein) is a cystine-knot like growth factor that can activate the canonical Wnt signaling pathway through FZD4 and LRP5/6 coreceptor. This group or ‘complex’ also includes molecules called glycosaminoglycans. Norrin plays a central role in retinal vascularization by acting as a ligand for FZD4 that signals via stabilizing beta-catenin (CTNNB1) and activating LEF/TCF-mediated transcriptional programs. The protein also acts in concert with TSPAN12 to activate FZD4 independently of the Wnt-dependent activation of FZD4, suggesting the existence of a Wnt-independent signaling that also promote accumulation the beta-catenin (CTNNB1). Norrin may be involved in a pathway that regulates neural cell differentiation and proliferation. Wnt signalling regulates multiple processes including angiogenesis, inflammation, and tumorigenesis. In humans, mutations in the gene that encodes Norrin can cause a disease in which blood vessels in the eye fail to form correctly, which can result in blindness. However, it is not clear how Norrin activates Wnt signalling.
From a laboratory at Cancer Research Technology.
| Product Type: | Protein |
| Name: | Norrin (Norrie disease protein, X-linked exudative viteoretinopathy 2 protein) |
| Accession ID: | Q00604 |
| Source: | Recombinant expression in HEK-293T Cells |
| Molecular Weight: | ~15 kDa (full length monomer) |
| Amino Acid Sequence: | KTDSSFIMDSDPRRCMRHHYVDSISHPLYKCSSKMVLLARCEGHCSQASRSEPLVSFSTVLKQPFRSSCHCCRPQTSKLKALRLRCSGGMRLTATYRYILSCHCEECNS |
| Fusion Tag(s): | C-terminal TETSQVAPA sequence derived from bovine rhodopsin (Rho-1D4) which is recognized by the Rho-1D4 monoclonal antibody |
| Purity: | Affinity and SEC purified |
| Buffer: | 10mM acetate buffer, pH 4.0, 0.5 M NaCl, 0.5% [w/v] CHAPS. (Can also be kept in 10 mM HEPES, pH 7.5, 0.7 M NaCl, 0.5% [w/v] CHAPS) |
| Concentration: | 25ug |
| Storage: | -80 °C; Multiple freeze/thaw cycles are not recommended |
| Shipped: | Dry ice |
Purification and Biological Activity
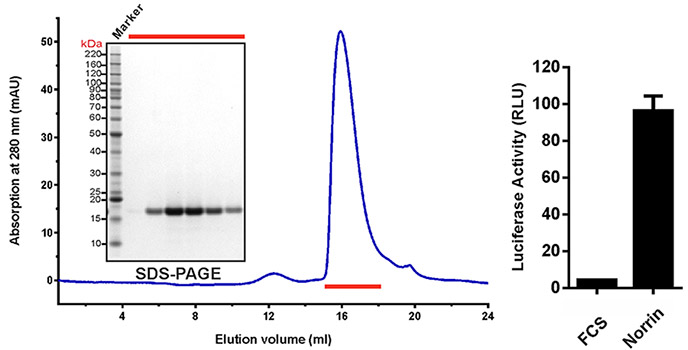
(Left) SEC elution profile and SDS-PAGE under reducing conditions with fraction analysed marked by red lines. (Right) Purified recombinant untagged Norrin actives the canonical Wnt/?-catenin pathway in the luciferase reporter assay. RLU: relative light unit. Error bars indicate standard deviations (n = 3).
Adapted from: Chang TH, et al. Elife. 2015 Jul 9;4. doi: 10.7554/eLife.06554.
- Chang TH, Hsieh FL, Zebisch M, Harlos K, Elegheert J, Jones EY. Structure and functional properties of Norrin mimic Wnt for signalling with Frizzled4, Lrp5/6, and proteoglycan. Elife. 2015 Jul 9;4. doi: 10.7554/eLife.06554.
If you publish research with this product, please let us know so we can cite your paper.