VSV-ΔG-GFP-2.6 Plasmid Expression Vector
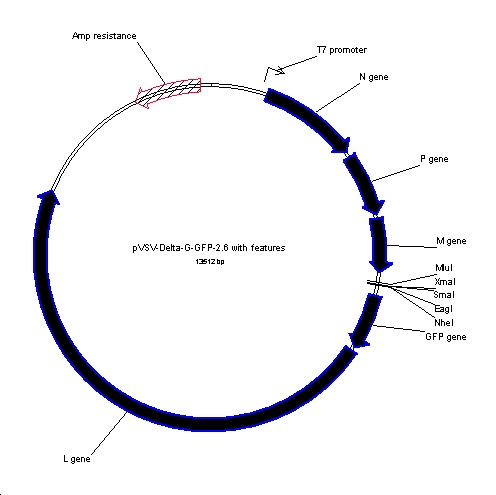
The plasmid pVSV-ΔG-GFP-2.6 encodes the antigenomic-sense (or positive-sense) RNA of a replication-restricted recombinant vesicular stomatitis virus (rVSV) in which the glycoprotein (G) gene has been replaced with a multiple cloning site (5'-MluI- XmaI-SmaI-EagI-NheI-3') and that contains an additional transcription unit between the MCS and the L gene that encodes GFP. This plasmid can be used to construct replication-competent viruses that encode a heterologous glycoprotein expressed from the MCS transcription unit. This plasmid is used together with plasmids encoding the VSV nucleocapsid (N), phosphoprotein (P), glycoprotein (G), and large polymerase subunit (L) to recovery rVSV as described in [1]. The antigenomic RNA of the virus is expressed from the bacteriophage T7 promoter in pBluescript, which has been further modified to contain the hepatitis delta ribozyme used to generate a precise 3' end of the VSV antigenomic RNA and a T7 terminator sequence cloned between the SacII and SacI restriction sites in pBS-SK+ [2, 3].
Recombinant vesicular stomatitis virusΔG (rVSV-ΔG) has been used to produce VSV pseudotypes containing the envelope glycoproteins of heterologous viruses including viruses that require high-level containment. Since the infectivity of rVSV-ΔG is restricted to a single round of replication, analyses of viral entry can be performed using just biosafety level 2 (BSL-2) containment.
It is the responsibility of the principal investigator to seek Institutional Biosafety Safety Committee approval for recombinant DNA, transgenic animal or infectious agent use within their laboratory spaces and maintain an Institutional Biosafety Safety Committee approval during the time period these materials are used.
From the laboratory of Michael A. Whitt, Ph.D., University of Tennessee.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
The plasmid pVSV-ΔG-GFP-2.6 encodes the antigenomic-sense (or positive-sense) RNA of a replication-restricted recombinant vesicular stomatitis virus (rVSV) in which the glycoprotein (G) gene has been replaced with a multiple cloning site (5'-MluI- XmaI-SmaI-EagI-NheI-3') and that contains an additional transcription unit between the MCS and the L gene that encodes GFP. This plasmid can be used to construct replication-competent viruses that encode a heterologous glycoprotein expressed from the MCS transcription unit. This plasmid is used together with plasmids encoding the VSV nucleocapsid (N), phosphoprotein (P), glycoprotein (G), and large polymerase subunit (L) to recovery rVSV as described in [1]. The antigenomic RNA of the virus is expressed from the bacteriophage T7 promoter in pBluescript, which has been further modified to contain the hepatitis delta ribozyme used to generate a precise 3' end of the VSV antigenomic RNA and a T7 terminator sequence cloned between the SacII and SacI restriction sites in pBS-SK+ [2, 3].
Recombinant vesicular stomatitis virusΔG (rVSV-ΔG) has been used to produce VSV pseudotypes containing the envelope glycoproteins of heterologous viruses including viruses that require high-level containment. Since the infectivity of rVSV-ΔG is restricted to a single round of replication, analyses of viral entry can be performed using just biosafety level 2 (BSL-2) containment.
It is the responsibility of the principal investigator to seek Institutional Biosafety Safety Committee approval for recombinant DNA, transgenic animal or infectious agent use within their laboratory spaces and maintain an Institutional Biosafety Safety Committee approval during the time period these materials are used.
From the laboratory of Michael A. Whitt, Ph.D., University of Tennessee.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
| Catalog Number | Product | DataSheet | Size | AVAILABILITY | Price | Qty |
|---|
| Product Type: | Plasmid |
| Alternative Name(s): | antigenomic-sense (or positive-sense) RNA of vesicular stomatitis virus (VSV) ΔG-GFP-2.6 |
| Gene/insert name: | rVSV-ΔG-GFP-2.6 |
| Antibiotic Resistance: | Ampicillin or Kanamycin (please see vial label and packing slip) |
| Fusion Tag(s): | GFP |
| Concentration: | 100uL (100ng/uL) |
| Amount: | 10 ul aliquot of plasmid in shipping vial |
| Grow in E. coli at 37 C: | Yes |
| Selectable markers: | N/A |
| Cloning Site 5': | 5' VSV sequence joined directly to T7 promoter |
| Cloning Site 3': | 3' VSV sequence joined directly to HDV ribozyme |
| Insert Size: | 10,670 bp |
| Vector Backbone and Size: | pBS-SK-ΦT, 3105 bp |
| High or low copy: | High |
| Comments: | For suggested protocol, see: Whitt, MA, J. Virol. Methods, 2010. 169(2): p. 365-74. |
| Shipped: | Ambient temperature |
VSV recovery requires BHK-21 cells and T7 supplied by vaccinia virus infection.
- Whitt, M.A., Generation of VSV pseudotypes using recombinant DeltaG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J. Virol. Methods, 2010. 169(2): p. 365-74.
- Lawson, N.D., et al., Recombinant vesicular stomatitis viruses from DNA. Proc.Natl.Acad.Sci.(USA), 1995. 92(10): p. 4477-4481.
- Stillman, E.A., J.K. Rose, and M.A. Whitt, Replication and amplification of novel vesicular stomatitis virus minigenomes encoding viral structural proteins. J. Virol., 1995. 69: p. 2946-2953.
- Lee SS, Phy K, Peden K, Sheng-Fowler L. Development of a micro-neutralization assay for ebolaviruses using a replication-competent vesicular stomatitis hybrid virus and a quantitative PCR readout. Vaccine. 2017 Apr 17. pii: S0264-410X(17)30325-0. View Article
If you publish research with this product, please let us know so we can cite your paper.


