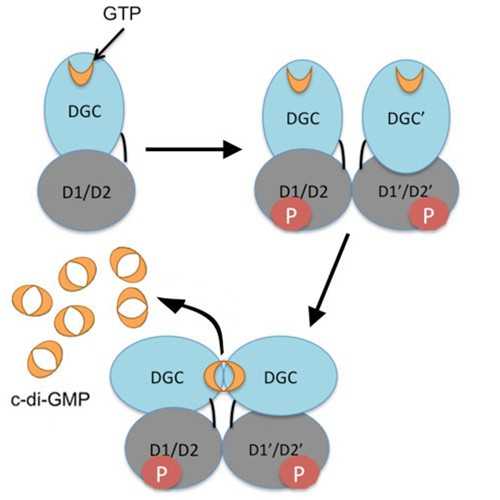
Engineered Diguanylate Cyclase
This diguanylate cyclase from Agrobacterium vitis has been engineered to remove phosphodiesterase activity, allowing for production of cyclic-diGMP from guanosine triphosphate (GTP) without the production of 5’-phosphoguanylyl-(3’,5’)-guanosine (pGpG).
Highlights:
- Useful for producing cyclic-diGMP from GTP without production of pGpG
- Completely lacks unwanted phosphodiesterase activity
- No product inhibition even at high concentrations of GTP
- Remains active while immobilized to solid resin and retain enzymatic activity after several months of storage
- Can be used to synthesize radiolabeled cyclic diGMP from radiolabeled GTP
- See Also: Cyclic Diguanosine Monophosphate (c-di-GMP)
Cyclic-diGMP is a second messenger used in signal transduction in bacteria, important in studies of bacterial virulence, bacterial infectiousness, pathogenesis, vaccine adjuvants (potentially in MRSA vaccines), innate immunity response, bacterial biofilm formation, bacterial cell-cell interactions, and bacterial cell adhesion. Biological activity regulated by cyclic-diGMP is mediated through binding to many different receptors. Additionally, it has been more recently shown that cyclic-diGMP induces the production of type I interferons in a STING or DDX41 dependent manner in the mammalian innate immune system.
From the laboratory of Elizabeth M. Boon, PhD, Stony Brook University.
This diguanylate cyclase from Agrobacterium vitis has been engineered to remove phosphodiesterase activity, allowing for production of cyclic-diGMP from guanosine triphosphate (GTP) without the production of 5’-phosphoguanylyl-(3’,5’)-guanosine (pGpG).
Highlights:
- Useful for producing cyclic-diGMP from GTP without production of pGpG
- Completely lacks unwanted phosphodiesterase activity
- No product inhibition even at high concentrations of GTP
- Remains active while immobilized to solid resin and retain enzymatic activity after several months of storage
- Can be used to synthesize radiolabeled cyclic diGMP from radiolabeled GTP
- See Also: Cyclic Diguanosine Monophosphate (c-di-GMP)
Cyclic-diGMP is a second messenger used in signal transduction in bacteria, important in studies of bacterial virulence, bacterial infectiousness, pathogenesis, vaccine adjuvants (potentially in MRSA vaccines), innate immunity response, bacterial biofilm formation, bacterial cell-cell interactions, and bacterial cell adhesion. Biological activity regulated by cyclic-diGMP is mediated through binding to many different receptors. Additionally, it has been more recently shown that cyclic-diGMP induces the production of type I interferons in a STING or DDX41 dependent manner in the mammalian innate immune system.
From the laboratory of Elizabeth M. Boon, PhD, Stony Brook University.
| Product Type: | Protein |
| Name: | Diguanylate cyclase (DGC); variant AVL from Nesbitt NM, et al. Biotechnology Reports. 2015; 7:30-37 |
| EC Number: | 2.7.7.65 |
| Accession ID: | P0AA89, ACM37222 |
| Source: | Agrobacterium vitisprotein over-expressed in Escherichia coli |
| Molecular Weight: | 56 kDa |
| Amino Acid Sequence: | mvnqpfevse pdridrltrr lereraarrq aetllearar elymancqla rqaedleqrv mqrteelvqe reraialaqq dqltglanrr sftavltdac qksrlrgdrv tvvlvdmdrf kaindayghe agdavlrefa rrlmvaagpg tvarlggdef alildhlsdv tadhafvssf raslqepvlf ngkrlevsas igyaslpdha asstellrha dmalyvskka glslatafdd dlwaeanerr llelelvlam drheimpwyq pivdgaskri lgvaalarwq hpyrglvlpg vflplaeern lldrlfaqva raacaqtapl vkagklnyvs vnispsqfks gnlamviasi leqtgfpata lvveitedli msdllragre lselsrlgvr valddfgtgy snisslsslp iqwlkldrsl isrvgddrvg qvivqavlqm aqalgidvva egietevqar wlveagcrkh qgflygrpap ledllcfsqp erlslaagks plehhhhhh |
| Fusion Tag(s): | C-terminal hexahistidine tag |
| Purity: | >99% based on SDS-PAGE analysis with coomassie blue |
| Buffer: | 50 mM Tris-HCl, pH 7.4, 5 mM β-mercaptoethanol, 10% glycerol, 50 mM arginine, 50 mM glutamic acid, 200 mM sodium chloride, 500 uM ethylenediaminetetraacetic acid (EDTA) and 10% glycerol. |
| Concentration: | 0.8mg/mL |
| Activity: | 7.5 nmol min-1 |
| Storage: | -80 °C; Multiple freeze/thaw cycles are not recommended |
| Shipped: | Dry ice |
Purification and Biological Activity
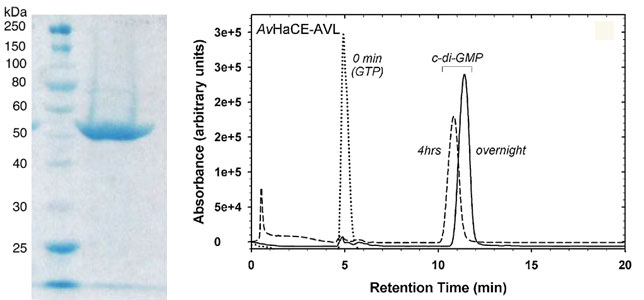
(Left) Coomassie blue stained SDS-PAGE gel (12.5%) of purified AvHaCE. Lane 1, molecular mass standards; lane 2, AvHaCE afterpurification by immobilized metal affinity chromatography with NiNTA as the matrix. (Right) Enzymatic synthesis of cyclic-diGMP with the AvHaCE AVL variant. HPLC analysis of reaction products at 0 min (dotted line), after 4 h incubation (dashed line), and overnight incubation (solidline) at room temperature. The species observed at 0 min is GTP.
Adapted from: Nesbitt NM, et al. Biotechnology Reports. 2015; 7:30-37.
Reactions can be run at 25 to 37 °C with50 mM TrisHCl, pH 7.5 containing 5 mM MgCl2 as the buffer
- Nesbitt NM, Arora DP, Johnson RA, Boon, EM. Modification of a bi-functional diguanylate cyclase-phosphodiesterase to efficiently produce cyclic di-guanylate monophosphate. Biotechnology Reports. 2015; 7:30-37.
If you publish research with this product, please let us know so we can cite your paper.