B Cell Activating Feeder Cell Line (EL-4-B5)
Cell Line EL-4-B5 is a subclone of murine EL4 thymoma that is a bromo-deoxyuridine-resistant mutant, and can be used as a feeder layer to generate B cell cultures yielding human monoclonal antibodies.
IL-2 producing EL-4 mouse thymoma cells underwent mutagenesis with ethyl-methane-sulfonate resulting in fusion partners for T cell hybridomas. It was then discovered that the thymidine kinase-deficient and ouabaine-resistant clone EL-4 BurOUr 6.1 also activated murine and human B cells via cell contact. Subcloning produced the EL-4-B5 cells, which strongly activate B cells. EL4-B5 is grown with B cells to activate the B cells via direct cell contact to induce proliferation, differentiation, and secretion of antibody. The EL-4-B5 cell line offers a method for the generation of recombinant human mAbs from single antigen-specific B cell clones selected with fluorescent VLPs and can be used to generate human mAbs to many other viruses whose proteins can self-assemble into VLPs.
From the laboratory of James E. Crowe, Jr., MD, Vanderbilt University.
Cell Line EL-4-B5 is a subclone of murine EL4 thymoma that is a bromo-deoxyuridine-resistant mutant, and can be used as a feeder layer to generate B cell cultures yielding human monoclonal antibodies.
IL-2 producing EL-4 mouse thymoma cells underwent mutagenesis with ethyl-methane-sulfonate resulting in fusion partners for T cell hybridomas. It was then discovered that the thymidine kinase-deficient and ouabaine-resistant clone EL-4 BurOUr 6.1 also activated murine and human B cells via cell contact. Subcloning produced the EL-4-B5 cells, which strongly activate B cells. EL4-B5 is grown with B cells to activate the B cells via direct cell contact to induce proliferation, differentiation, and secretion of antibody. The EL-4-B5 cell line offers a method for the generation of recombinant human mAbs from single antigen-specific B cell clones selected with fluorescent VLPs and can be used to generate human mAbs to many other viruses whose proteins can self-assemble into VLPs.
From the laboratory of James E. Crowe, Jr., MD, Vanderbilt University.
| Product Type: | Cell Line |
| Name: | EL-4-B5 |
| Cell Type: | Mouse thymoma continuous cell line |
| Accession ID: | CVCL_5I39 |
| Organism: | Mouse |
| Biosafety Level: | 2 |
| Growth Conditions: | RPMI-1640, 10% FBS heat inactivated, with 2 mM glutamine, usually antibiotics (typically pen/strep or gentamicin plus amphotericin B), 10 mM HEPES, 2- mercaptoethanol (0.056 mM) |
| Subculturing: | Cells are both adherent and suspension. The suspension cells are the more healthy ones. Can tolerate large splits after line is established (volume ratios of 1:10, for example). Helper function is optimal when cells are in log phase growth. Keep density below 106cells per mL at all times |
| Cryopreservation: | Standard conditions (typically 10% DMSO, 90% FBS) |
| Mycoplasma Tested: | Yes |
| Storage: | Liquid nitrogen |
| Shipped: | Dry ice |
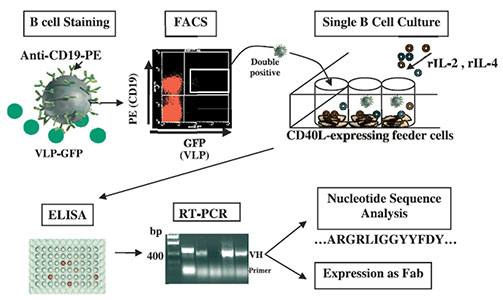
Human lymphocytes identified by flow cytometry to be double-positive for CD19-PE and RV VLP-GFP were single cell sorted into 96-well plates (one cell per well) and expanded in culture using a feeder-cell layer and cytokine combinations as indicated. Production of total Ig and RV VP6- or VP7-specific Ig was detected by ELISA using supernatants of the B cell cultures. Antibody VH and VL regions were rescued by nested RT-PCR followed by TA cloning. Nucleotide sequences were analyzed, unique VH and VL regions were subcloned into a novel Fab expression vector, and subsequently expressed as Fabs.
Adapted from: Weitkamp J-H., et al. J Immunol Methods 2003, 275:223-237.
Maintenance Tips from Producing Laboratory:
- It is not unusual at all for the vibrant culture counted just after thawing to take a dismal turn within a short 24-hours to what appears to be complete failure. Generally speaking, these cells may take a week or so, but the culture rebounds and becomes very difficult to keep up with because it grows so robustly. If in doubt, scrape the walls of the flask to remove the adherent phenotype; Stand the culture flask up in the incubator for ~1 hour, to allow cells to settle to the bottom; remove an arbitrary volume of medium from the 'top' of the culture, so that the total volume of medium is obviously reduced, forcing the cells closer together and hopefully stimulating the tactile contact that budding cultures need.
- Most lymphocytes in culture - in the same culture flask, exhibit two phenotypes: they float about in little Volvox-like balls or they pile on top of each other, with the cells on the bottom of the pile sticking to the plastic of the flask. The charge of the plastic is the accounts for this. There is no need to trypsinize the adherent cells, but they can be scraped gently with a cell scraper, if you want to bother with them at all. Once my cultures were established, the cells were generally had from the suspended ones. When using the cells or just passaging them, you can ignore the adherent ones.
- B cell helper function depends on the batch of FBS used. There is no simple way to identify optimal FBS lots, other than trial and error with the final assay (typically human B cell expansion).
- Zubler RH,Erard F,Lees RK,VanLaer M,Mingari C,Moretta L,MacDonald HR. Mutant EL-4 thymoma cells polyclonally activate murine and human B cells via direct cell interaction. J Immunol 1985, 134:3662-3668.
- Li W,Hanvanich M,Werner-Favre C,Brouwers N,Perrin LH,Zubler RH. Limiting dilution assay for human B cells based on their activation by mutant EL-4 thymoma cells: total and anti-malaria responder B cell frequencies. Eur J Immunol 1987, 17:887-892.
- Lundgren M,Persson U,Larsson P,Magnusson C,Smith CIE,Hammarstrom L, Severinson E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol 1989, 19 :1311-1315.
- Kwekkeboom J, De Boer M, Tager JM, De Groot C. CD40 plays an essential role inthe activation of human B cells by murine EL4B5 cells. Immunology. 1993Jul;79(3):439-44.
- Steenbakkers PG, Van Wezenbeek PM, van Zanten J, The TH. Efficient generation of human anti-cytomegalovirus IgG monoclonal antibodies from preselectedantigen-specific B cells. Hum Antibodies Hybridomas. 1993 Oct;4(4):166-73.
- Brinkmann V, Heusser CH. T cell-dependent differentiation of human B cellsinto IgM, IgG, IgA, or IgE plasma cells: high rate of antibody production by IgE plasma cells, but limited clonal expansion of IgE precursors. Cell Immunol. 1993 Dec;152(2):323-32.
- Matthes T,Werner-Favre C, Tang H, Zhang X, Kindler V, Zubler RH. Cytokine mRNA expression during an in vitro response of human B lymphocytes: Kinetics of tumor necrosis factor-a, interleukin (IL) 6, IL-10 and transforming growth factor b-1 mRNAs. J Exp Med 1993, 178:521-528.
- DeWildt RMT,Steenbakkers PG,Pennings AHM,vandenHoogen FHJ,van Venrooij WJ, Hoet RMA. A new method for the analysis and production of monoclonal antibody fragments originating from single human B cells. J Immunol Methods 1997, 207:61-67
- Leyendeckers H, Odendahl M, Lohndorf A, Irsch J, Spangfort M, Miltenyi S, Hunzelmann N, Assenmacher M, Radbruch A, Schmitz J. Corelation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur J Immunol 1999, 29:1406-1417.
- Weitkamp J-H, Kallewaard N, Kusuhara K, Feiglstock D, Feng N, Greenberg HB, Crowe JE Jr. Generation of recombinant human monoclonal antibodies to rotavirus from single antigen-specific B cells selected with fluorescent virus-like particles. J Immunol Methods 2003, 275:223-237.
If you publish research with this product, please let us know so we can cite your paper.