IDG-SW3 Bone Cell Line
Cell line IDG-SW3 replicates osteoblast to late osteocyte differentiation in vitro and increases bone formation in vivo.
Highlights:
- Express series markers from early to late osteocytes, including: Dmp1-GFP, E11/gp38, SOST/sclerostin, and FGF-23
- Maintained in non-differentiated state at 33°C with IFN-g present - allows for large scale production without the loss of phenotype
- Same gene expression as primary cells when cultured at 37°C with no IFN-g present
- Clonal cells - homogeneous, and at the same stage of differentiation
- Can be maintained at both 2D and 3D cultures
- Long term viability (35-50 days)
Osteocytes are the most abundant bone cells in the body but also the most challenging to study because they are embedded in a mineralized matrix making them to difficult to isolate. This cell line should be useful study osteoblast to osteocyte transition, mechanisms for biomineralization osteocyte function and regulation of SOST/sclerostin and FGF-23.
From the laboratory of Lynda F. Bonewald, PhD, University of Missouri - Kansas City.
Cell line IDG-SW3 replicates osteoblast to late osteocyte differentiation in vitro and increases bone formation in vivo.
Highlights:
- Express series markers from early to late osteocytes, including: Dmp1-GFP, E11/gp38, SOST/sclerostin, and FGF-23
- Maintained in non-differentiated state at 33°C with IFN-g present - allows for large scale production without the loss of phenotype
- Same gene expression as primary cells when cultured at 37°C with no IFN-g present
- Clonal cells - homogeneous, and at the same stage of differentiation
- Can be maintained at both 2D and 3D cultures
- Long term viability (35-50 days)
Osteocytes are the most abundant bone cells in the body but also the most challenging to study because they are embedded in a mineralized matrix making them to difficult to isolate. This cell line should be useful study osteoblast to osteocyte transition, mechanisms for biomineralization osteocyte function and regulation of SOST/sclerostin and FGF-23.
From the laboratory of Lynda F. Bonewald, PhD, University of Missouri - Kansas City.
| Catalog Number | Product | DataSheet | Size | AVAILABILITY | Price | Qty |
|---|
| Product Type: | Cell Line |
| Name: | IDG-SW3 |
| Cell Type: | Osteoblast/early osteocyte/late osteocyte |
| Accession ID: | CVCL_0P23 |
| Organism: | Mouse |
| Source: | Long bone |
| Morphology: | Adherent osteoblast-like cell |
| Biosafety Level: | I |
| Subculturing: |
See: |
| Growth Conditions: |
Proliferation medium: AlphaMEM (containing L-glutamine and deoxyribonucleosides); supplemented with 10% FBS; 1% penicillin-streptomycin; Recombinant Mouse Interferon-gamma (INF-γ) 50 U/ml. Grown on dishes coated with [0.15 mg/ml] rat tail type I collagen. Incubate at 33°C with 5% CO2 Differentiation medium: AlphaMEM (L-glutamine and deoxyribonucleosides); supplemented with 10% FBS; 1% penicillin-streptomycin; 50µg/ml Ascorbic Acid and 4mM β-glycerophosphate. Grown on dishes coated with [0.15 mg/ml] rat tail type I collagen. Incubate at 37°C with 8% CO2. |
| Cryopreservation: | 60% AlphaMEM, 30% FBS, 10% DMSO, at 1-2 x 10^6 cells/vial/1ml |
| Storage: | Liquid nitrogen |
| Shipped: | Dry ice |
Osteoblastic and osteocytic markers
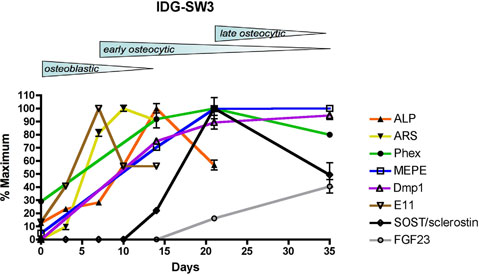
Schematic diagram summarizing ostoblastic and osteocytic markers in IDG-SW3 cells over time. IDG-SW3 cells transition from late osteoblasts to late osteocytes in vitro on both 2D and 3D substrates.
Adapted from: Woo SM. et al. J Bone Miner Res. 2011 Nov;26(11):2634-46.
Experimental Protocol:
- For proliferation/expansion: incubate at 33°C with 5% CO2
- For differentiation: incubate at 37°C with 8% CO2
- Plate the cells at a density of 4x10^4 cells/cm2, in proliferation medium and incubate at 33°C, 8% CO2 , until confluent, called Day 0.
- At Day 0, switch to differentiation medium, and incubate at 37°C, 8% CO2, exchanging media every 2-3 days.
- The GFP expression, controlled by the DMP1 promoter, is usually observed at 3-4 days of culture under differentiation conditions, and should be strong by days 10-14 of culture.
NOTE: Mineralization and gene expression may be Fetal Bovine Serum dependent; testing and optimization of different serum lots/batches may be necessary. Mineralization and gene expression may be CO2 dependent; testing and optimization of 5-10% CO2 may be necessary. Testing with our current serum showed that differentiation of the IDG-SW3 in 8% CO2 resulted in an increase of mineral, and in SOST and DMP1 gene expression, when compared to cells differentiated in 5% CO2.
- Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res. 2011 Nov;26(11):2634-46.
- Ito N, Findlay DM, Anderson PH, Bonewald LF, Atkins GJ. Extracellular phosphate modulates the effect of 1?,25-dihydroxy vitamin D3 (1,25D) on osteocyte like cells J Steroid Biochem Mol Biol. 2013 Jul;136:183-6.
- St John HC, Bishop KA, Meyer MB, Benkusky NA, Leng N, Kendziorski C, Bonewald LF, Pike JW. The osteoblast to osteocyte transition: epigenetic changes and response to the vitamin d3 hormone. Mol Endocrinol. 2014 Jul;28(7):1150-65.
- Fujita K, Xing Q, Khosla S, Monroe DG. Mutual Enhancement of Differentiation of Osteoblasts and Osteocytes Occurs Through Direct Cell-Cell Contact. J Cell Biochem. 2014 Jul 15.
- Shao J, Zhou Y, Lin J, Nguyen TD, Huang R, Gu Y, Friis T, Crawford R, Xiao Y. Notch expressed by osteocytes plays a critical role in mineralisation. J Mol Med (Berl). 2018 Feb 17. View Article
- Shao J, Zhou Y, Xiao Y. The regulatory roles of Notch in osteocyte differentiation via the crosstalk with canonical Wnt pathways during the transition of osteoblasts to osteocytes. Bone. 2018 Mar;108:165-178. View Article
- Giacomino CM, Wealleans JA, Kuhn N, Diogenes A. Comparative Biocompatibility and Osteogenic Potential of Two Bioceramic Sealers. J Endod. 2019 Jan;45(1):51-56. View Article
- Bär L, Hase P, Föller M. PKC regulates the production of fibroblast growth factor 23 (FGF23). PLoS One. 2019 Mar 28;14(3):e0211309. View Article
- Zhou Y, Lin J, Shao J, Zuo Q, Wang S, Wolff A, Nguyen DT, Rintoul L, Du Z, Gu Y, Peng YY, Ramshaw JAM, Long X, Xiao Y. Aberrant activation of Wnt signaling pathway altered osteocyte mineralization. Bone. 2019 Jun 28;127:324-333. View Article
- Hingorani DV, Camargo MF, Quraishi MA, Adams SR, Advani SJ. Tumor Activated Cell Penetrating Peptides to Selectively Deliver Immune Modulatory Drugs. Pharmaceutics. 2021 Mar 10;13(3):365. View article
If you publish research with this product, please let us know so we can cite your paper.