pJA15-Template for in vitro tRNA expression
This plasmid contains the T7 RNA polymerase promoter and E. coli tRNA His and is used as a template for in vitro transcription of E. coli tRNA His. The tRNA gene of interest can be inserted into the multiple cloning site of the expression plasmid, downstream from the T7 RNA promoter. The tRNA gene terminates with a FokI restriction site, such that run-off transcription of the linearized plasmid produces a tRNA transcript with the correct CCA end.
From the laboratory of Christopher S. Francklyn, PhD, University of Vermont.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
This plasmid contains the T7 RNA polymerase promoter and E. coli tRNA His and is used as a template for in vitro transcription of E. coli tRNA His. The tRNA gene of interest can be inserted into the multiple cloning site of the expression plasmid, downstream from the T7 RNA promoter. The tRNA gene terminates with a FokI restriction site, such that run-off transcription of the linearized plasmid produces a tRNA transcript with the correct CCA end.
From the laboratory of Christopher S. Francklyn, PhD, University of Vermont.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
| Product Type: | Plasmid |
| Gene/insert name: | T7 RNA polymerase promoter + E.coli tRNA His |
| Accession ID: | AAC75567, P12081 |
| Antibiotic Resistance: | Ampicillin |
| Fusion Tag(s): | none |
| Grow in E. coli at 37 C: | Yes |
| Selectable markers: | Ampicillin |
| Cloning Site 5': | EcoRI |
| Cloning Site 3': | BamHI |
| Insert Size: | 115 bp |
| Vector Backbone and Size: | pUC19, 2686 bp |
| High or low copy: | High |
| Shipped: | Ambient temperature (Liquid plasmid DNA in water for domestic orders, spotted on filter paper for international orders) |
Linearize plasmid with FokI prior to in vitro transcription as described in Francklyn et. al, Methods, 2008
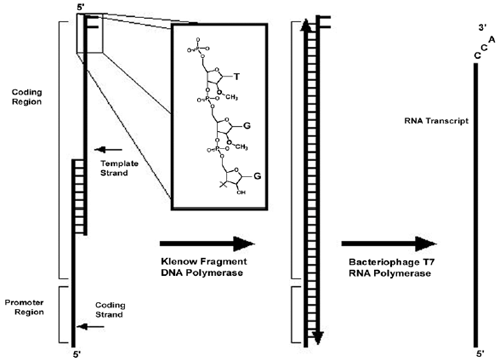 |
| Schematic of synthetic template transcription. Overlapping synthetic nucleotides are used as a template for Klenow fragment extension by annealing and extension cycles. The template strand contains 2'-O-methyl modifications on the two terminal 5' residues (inset). The doublestranded fragment then acts as a template for T7 RNA polymerase. The DNA modifications are thought to cause the polymerase to dissociate from the template before adding nontemplated residues, thereby increasing product homogeneity. Adapted from: Francklyn et. al, Methods, Vol 44, No.2, pp 100-118, 2008. |
- Arnez, J.G., Harris, D.C., Mitschler, A., Reese, B., Francklyn, C.S., and Moras, D. Crystal structure of histidyl-tRNA synthetase from Escherichia coli complexed with histidyl-adenylate. EMBO Journal, 14:17, 4143-4155 (1995).
- Yan, W., Augustine, J., and Francklyn, C. A tRNA identity switch mediated by the binding interaction between a tRNA anticodon and the accessory domain of a class II aminoacyl-tRNA synthetase. Biochemistry, 35:21, 6559-6568 (1996).
- Arnez, J.G., Augustine, J.G., Moras, D., and Francklyn, C. The first step of aminoacylation at the atomic level in histidyl-tRNA synthase. PNAS, 94, 7144-7149 (1997).
- Francklyn, C., Musier-Forsyth, K., and Martinis, S.A. Aminoacyl-tRNA synthetases in biology and disease: new evidence for structural and functional diversity in an ancient family of enzymes RNA, 3, 945-960 (1997).
- Francklyn, C., Adams, J., and Augustine, J. Catalytic defects in mutants of class II histidyl-tRNA synthetase from Salmonella typhimurium previously linked to decreased control of histidine biosynthesis regulation. Journal of Molecular Biology, 280, 847-858 (1998).
- Hawko, S.A., and Francklyn, C.S. Covariation of a specificity-determining structural motif in an aminoacyl-tRNA synthetase and a tRNA identity element. Biochemistry, 40:7, 1930-1936 (2001).
- Connoly, S.A., Rosen, A.E., Musier-Forsyth, K., and Francklyn, C.S. G-1:C73 recognition by an arginine cluster in the active site of Escherichia coli histidyl-tRNA synthetase. Biochemistry, 43: 4, 962-969 (2004).
- Guth, E., Connoly, S.H., Bovee, M., and Francklyn, C.S. A substrate-assisted concerted mechanism for aminoacylation by a class II aminoacyl-tRNA synthetase. Biochemistry, 44:10, 3785-3794 (2005)
- Guth, E. and Francklyn, C.S. Kinetic discrimination of tRNA identity by the conserved motif 2 loop of a class II aminoacyl-tRNA synthetase. Molecular Cell, 25, 531-542 (2007).
- Francklyn, C.S., First, E.A., Perona, J.J., and Hou, Y. Methods for kinetic and thermodynamic analysis of aminoacyl-tRNA synthetases. Methods, 44:2, 100-118 (2008).
If you publish research with this product, please let us know so we can cite your paper.