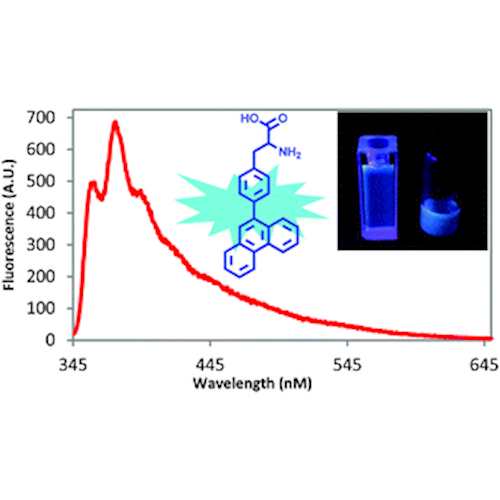
Fluorescent Phenylalanine Analog (Phen-AA)
This novel fluorescent L-α-amino acid 4-phenanthracen-9-yl-L-phenylalanine (Phen-AA) emits greenish blue light in the visible region. The genetically encodable L-α-amino acid may find broad applications in research, biotechnology, and the pharmaceutical industry.
Highlights:
- Excellent photostability with a 75% quantum yield
- Its synthetic procedure (involving three simple steps and mild conditions) is resistant to racemization
- Readily gets into human cells, being imaged clearly upon 405 nm laser excitation
Alpha-amino acids are the common natural forms of amino acids. They are simple molecules, containing a primary amine group (NH2) and a carboxylic group (COOH) separated by one carbon (alpha-carbon). Except for glycine, other natural amino acids adopt the L configuration. Phenylalanine is an essential α-amino acid. It is used in the manufacture of food and drink products and sold as a nutritional supplement for its reputed analgesic and antidepressant effects. The L-isomer is used to biochemically form proteins, coded for by DNA.
From the laboratory of Maolin Guo, PhD, University of Massachusetts Dartmouth.
This novel fluorescent L-α-amino acid 4-phenanthracen-9-yl-L-phenylalanine (Phen-AA) emits greenish blue light in the visible region. The genetically encodable L-α-amino acid may find broad applications in research, biotechnology, and the pharmaceutical industry.
Highlights:
- Excellent photostability with a 75% quantum yield
- Its synthetic procedure (involving three simple steps and mild conditions) is resistant to racemization
- Readily gets into human cells, being imaged clearly upon 405 nm laser excitation
Alpha-amino acids are the common natural forms of amino acids. They are simple molecules, containing a primary amine group (NH2) and a carboxylic group (COOH) separated by one carbon (alpha-carbon). Except for glycine, other natural amino acids adopt the L configuration. Phenylalanine is an essential α-amino acid. It is used in the manufacture of food and drink products and sold as a nutritional supplement for its reputed analgesic and antidepressant effects. The L-isomer is used to biochemically form proteins, coded for by DNA.
From the laboratory of Maolin Guo, PhD, University of Massachusetts Dartmouth.
| Product Type: | Small Molecule |
| Name: | 4-phenanthracen-9-yl-L-phenylalanine (Phen-AA) |
| Chemical Formula: | C23H19NO2 |
| Molecular Weight: | 341.14 |
| Format: | Off white powder |
| Purity: | >95% |
| Solubility: | Soluble in DMSO |
| Spectral Information: | See reference: Chem. Commun., 2020,56, 12578-12581 |
| Quantum Yield: | 75% |
| Comments: | Usually dissolve in DMSO first, then dilute it in a desired solvent or solvent mixture. The provider recommends 2mg per experiment. |
| Storage: | -20C or -80C |
| Shipped: | Dry ice |
- Gupta A , Garreffi BP , Guo M . Facile synthesis of a novel genetically encodable fluorescent Α-amino acid emitting greenish blue light. Chem Commun (Camb). 2020 Oct 25;56(83):12578-12581. doi: 10.1039/d0cc03643a. Epub 2020 Sep 18. PMID: 32944728; PMCID: PMC7577945.
If you publish research with this product, please let us know so we can cite your paper.