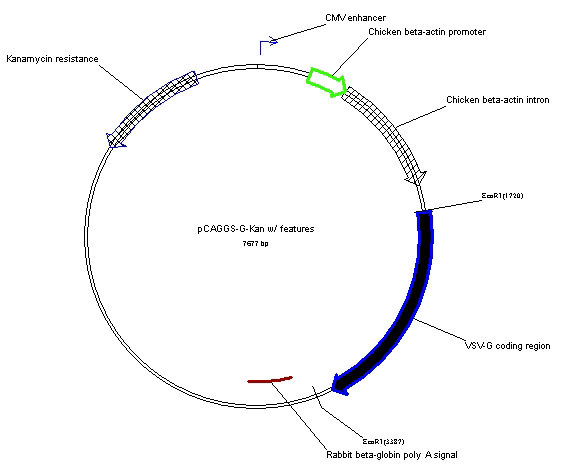
pCAGGS-G-Kan Plasmid
pCAGGS-G-Kan is a eukaryotic expression vector derived from pCAGGS [1] that has a cytomegalovirus (CMV) enhancer, the strong chicken ß-actin promoter followed by a chicken ß-actin intron sequence which drives expression of cDNAs inserted into the downstream of a multiple cloning site. pCAGGS-G-Kan contains the cDNA for the vesicular stomatitis virus (VSV) glycoprotein (G) isolated from the San Juan strain of the Indiana serotype of VSV. The plasmid can be used for transient expression of VSV-G in many different cells types. It is also used to pseudotype recombinant, replication-restricted ΔG-VSV as described [2]. This plasmid encodes kanamycin resistance due to replacement of the ß-lactamase gene in the original pCAGGS with the gene for neomycin phosphotransferase II.
From the laboratory of Michael A. Whitt, Ph.D., University of Tennessee.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
pCAGGS-G-Kan is a eukaryotic expression vector derived from pCAGGS [1] that has a cytomegalovirus (CMV) enhancer, the strong chicken ß-actin promoter followed by a chicken ß-actin intron sequence which drives expression of cDNAs inserted into the downstream of a multiple cloning site. pCAGGS-G-Kan contains the cDNA for the vesicular stomatitis virus (VSV) glycoprotein (G) isolated from the San Juan strain of the Indiana serotype of VSV. The plasmid can be used for transient expression of VSV-G in many different cells types. It is also used to pseudotype recombinant, replication-restricted ΔG-VSV as described [2]. This plasmid encodes kanamycin resistance due to replacement of the ß-lactamase gene in the original pCAGGS with the gene for neomycin phosphotransferase II.
From the laboratory of Michael A. Whitt, Ph.D., University of Tennessee.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
| Product Type: | Plasmid |
| Gene/insert name: | VSV-G (San Juan, Indiana serotype) |
| Antibiotic Resistance: | Ampicillin or Kanamycin (please see vial label and packing slip) |
| Fusion Tag(s): | none |
| Concentration: | 100uL (100ng/uL) |
| Grow in E. coli at 37 C: | Yes |
| Cloning Site 5': | EcoRI |
| Cloning Site 3': | EcoRI |
| Insert Size: | 1,673 bp |
| Vector Backbone and Size: | pCAGGS-Kan, 6004 bp |
| High or low copy: | High |
| Comments: | For suggested protocol, see: Whitt, MA, J. Virol. Methods, 2010. 169(2): p. 365-74. |
| Shipped: | Ambient temperature |
- Niwa, H., K. Yamamura, and J. Miyazaki, Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene, 1991. 108: p. 193-200.
- Whitt, M.A., Generation of VSV pseudotypes using recombinant DeltaG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J. Virol. Methods, 2010. 169(2): p. 365-74.
- Howell KA, Qiu X, Brannan JM, Bryan C, Davidson E, Holtsberg FW, Wec AZ,Shulenin S, Biggins JE, Douglas R, Enterlein SG, Turner HL, Pallesen J, Murin CD,He S, Kroeker A, Vu H, Herbert AS, Fusco ML, Nyakatura EK, Lai JR, Keck ZY, FoungSK, Saphire EO, Zeitlin L, Ward AB, Chandran K, Doranz BJ, Kobinger GP, Dye JM,Aman MJ. Antibody Treatment of Ebola and Sudan Virus Infection via a UniquelyExposed Epitope within the Glycoprotein Receptor-Binding Site. Cell Rep. 2016 May17;15(7):1514-26. View Article
- Almahboub SA, Algaissi A, Alfaleh MA, ElAssouli MZ, Hashem AM. Evaluation of Neutralizing Antibodies Against Highly Pathogenic Coronaviruses: A Detailed Protocol for a Rapid Evaluation of Neutralizing Antibodies Using Vesicular Stomatitis Virus Pseudovirus-Based Assay. Front Microbiol. 2020 Sep 4;11:2020. View Article
- Farzani TA, Chov A, Herschhorn A. A protocol for displaying viral envelope glycoproteins on the surface of vesicular stomatitis viruses. STAR Protoc. 2020 Dec 9;1(3):100209. View article
- Condor Capcha JM, Lambert G, Dykxhoorn DM, Salerno AG, Hare JM, Whitt MA, Pahwa S, Jayaweera DT, Shehadeh LA. Generation of SARS-CoV-2 Spike Pseudotyped Virus for Viral Entry and Neutralization Assays: A 1-Week Protocol. Front Cardiovasc Med. 2021 Jan 15;7:618651. View article
If you publish research with this product, please let us know so we can cite your paper.