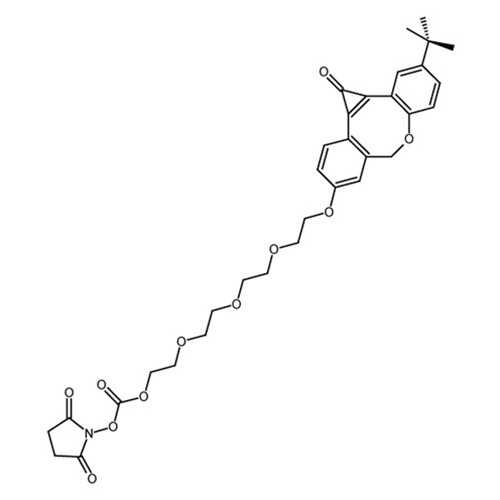
Photo-ODIBO-EG4-NHS
Photo-ODIBO-EG4-NHS for strain-promoted alkyne-azide cycloaddition (SPAAC) applications.
Highlights:
- The only reagent on the market that permits photo-SPAAC ligation
- Especially engineered for the production of particles and surfaces with spatially resolved chemical functionality, of interest to many areas of science and technology including the fabrication of biochips, microfluidic devices, targeted drug delivery, MEMS and NEMS
- Photo-ODIBO moiety does not react with organic or inorganic azides, thiols, water, and other endogenous nucleophiles, even at elevated temperatures
- Equipped with amine-reactive NHS group for easy derivatization of peptides, proteins, and tissues
- Upon mild UVA irradiation releases azide-reactive ODIBO, the most reactive SPAAC reagents on the market
- Permits spatially-resolved conjugation using SPAAC, SPANC, SPANOC, IEDDA, and thiol-yne conjugations
- Long shelf life in the dark
Precursors in the study of strain-promoted alkyne-azide cycloaddition (SPAAC), SPANC, Diels-Alder, and thiol-yne reactions cell and tissue labeling, surface modifiers, chemical synthesis, homo- and hetero-nuclear π metastasis reactions, light-selective labeling, photo-patterning, triazole synthesis, azide quenching reactions.
From the laboratory of Vladimir V. Popik, PhD, University of Georgia.
Photo-ODIBO-EG4-NHS for strain-promoted alkyne-azide cycloaddition (SPAAC) applications.
Highlights:
- The only reagent on the market that permits photo-SPAAC ligation
- Especially engineered for the production of particles and surfaces with spatially resolved chemical functionality, of interest to many areas of science and technology including the fabrication of biochips, microfluidic devices, targeted drug delivery, MEMS and NEMS
- Photo-ODIBO moiety does not react with organic or inorganic azides, thiols, water, and other endogenous nucleophiles, even at elevated temperatures
- Equipped with amine-reactive NHS group for easy derivatization of peptides, proteins, and tissues
- Upon mild UVA irradiation releases azide-reactive ODIBO, the most reactive SPAAC reagents on the market
- Permits spatially-resolved conjugation using SPAAC, SPANC, SPANOC, IEDDA, and thiol-yne conjugations
- Long shelf life in the dark
Precursors in the study of strain-promoted alkyne-azide cycloaddition (SPAAC), SPANC, Diels-Alder, and thiol-yne reactions cell and tissue labeling, surface modifiers, chemical synthesis, homo- and hetero-nuclear π metastasis reactions, light-selective labeling, photo-patterning, triazole synthesis, azide quenching reactions.
From the laboratory of Vladimir V. Popik, PhD, University of Georgia.
Specifications
| Product Type: | Small Molecule |
| Name: | Photo-ODIBO-EG4-NHS; Photo-ODIBO-TEG-NHS |
| Alternative Name(s): | 2-(2-(2-(2-((3-(tert-butyl)-1-oxo-1,7- dihydrodibenzo[b,f]cyclopropa[d]oxocin-9- yl)oxy)ethoxy)ethoxy)ethoxy)ethyl (2,5-dioxopyrrolidin- 1-yl) carbonate |
| Chemical Formula: | C33H37NO11 |
| CAS number: | 2095777-51-6 |
| Molecular Weight: | 623.65 |
| Format: | Colorless oil |
| Purity: | >99% 1HNMR |
| Solubility: | CH3CN, MeOH, etc. |
| Spectral Information: |
1H-NMR: 77.93-7.95 (m, 2H), 7.49-7.52 (dd, J = 8.5, 2.5 Hz,1H), ] 7.19-7.21 (d,J = 8.5 Hz,1H), 7.04-7.07 (m, 2H), 5.25-5.28 (d,J = 12.2 Hz, 1H), 4.77-4.80 (d, J = 12.2 Hz, 1H), 4.44-4.46 (m, 2H), 4.23-4.25 (t, J = 4.8 Hz, 2H), 3.90-3.92 (t, J = 4.8 Hz, 2H), 3.73-3.79 (m, 4H), 3.65-3.71 (m, 6H), 2.82 (s, 4H), 1.35 (s, 9H). 13C-NMR: 168.72, 162.19, 160.53, 152.81, 151.81, 148.13, 144.18, 142.35, 140.78, 135.61, 131.21, 130.80, 122.14, 117.99, 117.52, 117.47, 117.19, 114.82, 78.88, 71.09, 70.95, 70.81, 70.43, 69.62, 68.52, 68.14, 34.75, 31.52, 25.64. ESI HRMS: calcd. (M+H+): C33H38NO11+: 624.2439, found 624.2442. 13C-NMR: 1846 cm-1 (v C=O). |
| Storage: | +10C, Protect from light |
| Shipped: | Ambient Temperature |
Provider
From the laboratory of Vladimir V. Popik, PhD, University of Georgia.
References
- Matthew Bjerknes, Hazel Cheng, Christopher D. McNitt, and Vladimir V.Popik: Facile Quenching and Spatial Patterning of Cylooctynes via Strain-Promoted Alkyne–Azide Cycloaddition of Inorganic Azides. Bioconjugate Chem., 2017, 28 (5), pp 1560–1565
- Orski, SV, Poloukhtine, AA, Arumugam, S, Mao, L, Popik, VV, Locklin, J: High density orthogonal surface immobilization via photoactivated copper-free click chemistry: (2010) J. of the Am. Chem. Soc. 132(32) 11024-11026
- Selvanathan Arumugam, Sara V. Orski, Ngalle Eric Mbua, Christopher McNitt, Geert-Jan Boons, Jason Locklin, and Vladimir V. Popik: Photo-click chemistry strategies for spatiotemporal control of metal-free ligation, labeling, and surface derivatization. Pure Appl. Chem., Vol. 85, No. 7, pp. 1499–1513, (2013)
- US Patents US8,258,347, US8,426,649 and US8,541,625
If you publish research with this product, please let us know so we can cite your paper.