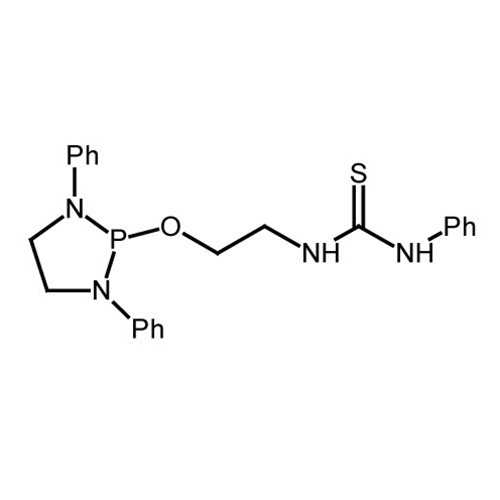
NHP-Thiourea
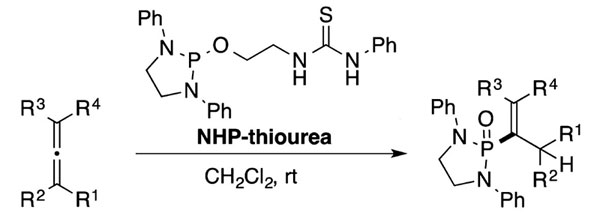
A versatile phosphonylation reagent which generates a phosphorus-carbon (P-C) bond with various electrophiles without metals under environmentally friendly and mild reaction conditions.
Highlights:
- Can be used in phospha-Michael reaction, phospha-Mannich reaction, and phospha-aldol reaction to form P-C bonds without the use of additives or bases
- Allows for one-step synthesis of various phosphonate compounds (e.g., vinylphosphonates, hydrazine phosphonates, α-aminophosphonates, α-hydroxyphosphonates, β-aminophosphonates, and γ-ketophosphonates)
- Metal-free, mild reaction conditions
Reducing the number of synthetic steps and removal of impurities such as toxic metal catalysts from drugs are challenging tasks within the pharmaceutical industry, and are significantly related to price deviations. The transition-metal-free synthetic route presents a green and cost-effective method.
From the laboratory of Jun Yong Kang, PhD, University of Nevada Las Vegas.
A versatile phosphonylation reagent which generates a phosphorus-carbon (P-C) bond with various electrophiles without metals under environmentally friendly and mild reaction conditions.
Highlights:
- Can be used in phospha-Michael reaction, phospha-Mannich reaction, and phospha-aldol reaction to form P-C bonds without the use of additives or bases
- Allows for one-step synthesis of various phosphonate compounds (e.g., vinylphosphonates, hydrazine phosphonates, α-aminophosphonates, α-hydroxyphosphonates, β-aminophosphonates, and γ-ketophosphonates)
- Metal-free, mild reaction conditions
Reducing the number of synthetic steps and removal of impurities such as toxic metal catalysts from drugs are challenging tasks within the pharmaceutical industry, and are significantly related to price deviations. The transition-metal-free synthetic route presents a green and cost-effective method.
From the laboratory of Jun Yong Kang, PhD, University of Nevada Las Vegas.
Specifications
| Product Type: | Small Molecule |
| Name: | 1-(2-((1,3-diphenyl-1,3,2-diazaphospholidin-2-yl)oxy)ethyl)-3-phenylthiourea |
| Chemical Formula: | C23H25N4OPS |
| Molecular Weight: | 436 |
| Format: | White solid |
| Purity: | >95%, 31P NMR |
| Solubility: | CH2Cl2, CHCl3, EtOAc |
| Spectral Information: | 1H NMR, 13C NMR, 31P NMR |
| Storage: | +2-4C under argon |
| Shipped: | Room temperature |
Research
Provider
From the laboratory of Jun Yong Kang, PhD, University of Nevada Las Vegas.
References
- Mulla, K.; Aleshire, K. L.;Forster, P. M. "Utility of Bi-Functional N-Heterocyclic Phosphine (NHP)-Thioureas for Metal-Free Carbon-Phosphorus Bond Construction toward Regio-, and Stereoselective Formation of Vinylphosphonates" Kang, J. Y. J. Org. Chem. 2016, 81, 77-88.
- Mulla, K.; Kang, J. Y. "1,3,2-Diazaphospholidine (N-Heterocyclic Phosphine)-Mediated Carbon-Phosphorus Bond Forming One-Pot Tandem Reaction: A Route to α-amino Phosphonates" J. Org. Chem. 2016, 81, 4550-4558.
- Huang, H.; Kang, J. Y. "Amine-Catalyzed Phospha-Michael Reaction of α, β-Unsaturated Aldehydes and Ketones with Multifunctional N-Heterocyclic Phosphine-Thioureas as Phosphonylation Reagent" Org. Lett. 2016, 18, 4372-4375.
- Moletti, N.; Kang, J. Y. "Catalyst-free synthesis of α1-oxindole- α-hydroxyphosphonates via phospha-aldol reaction of isatins employing N-heterocyclic phosphine (NHP)-thiourea" Org. Biomol. Chem. 2016, 14, 8952-8956.
- Moletti, N.; Bjornberg, C.; Kang, J. Y. "Phospha-Michael addition reaction of maleimides employing N-heterocyclic phosphine-thiourea as a phosphonylation reagent: synthesis of 1-aryl-2,5-dioxopyrrolidine-3-yl-phosphonate derivatives" Org. Biomol. Chem. 2016, 14, 10695-10704.
- Huang, H.; Palmas, J.; Kang, J. Y. "A Reagent-Controlled Phospha-Michael Addition Reaction of Nitroalkenes with Bifunctional N-Heterocyclic Phosphine (NHP)-Thioureas" J. Org. Chem. 2016, 81, 11932-11939.
- Moletti, N.; Kang, J. Y. "Synthesis of Diaryl-Diazaphosphonates via 1,6-Hydrophosphonylation of p-Quinone Methides with N-Heterocyclic Phosphine-Thioureas" Org. Lett. 2017, 19, 958-961.
If you publish research with this product, please let us know so we can cite your paper.