Streptavidin Mutein Matrix SAVSBPM18
Streptavidin SBPM18 is a streptavidin mutein that can bind both biotinylated proteins and SBP tagged proteins with high affinity (Kd ~10-8M) in a reversible manner.
Highlights:
- Provided as SBPM18 mutein-Sepharose CL-6B affinity matrix
- Ideal for affinity purification of both biotinylated biomolecules and recombinant proteins tagged with streptavidin binding peptide (SBP) tags
- Target proteins can be affinity purified in high purity and with excellent recovery
- Mild conditions are used for elution of target proteins and regeneration of the column
- Many buffers can be used for in the binding, washing and elution steps
- The affinity matrix is reusable
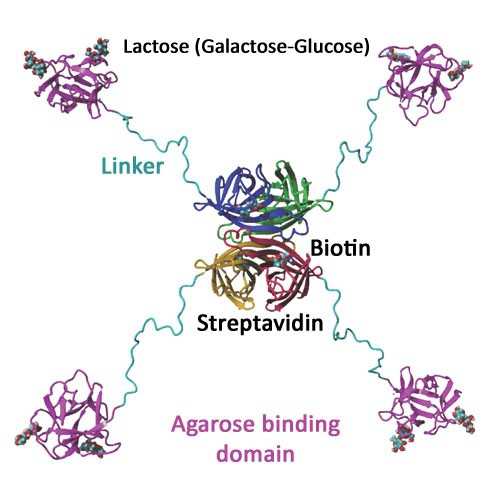
Streptavidin binding peptide (SBP) tag is a peptide tag that binds to streptavidin with nmolar binding affinity (2). Although the original peptide tag is 38 amino acids in length, crystallographic and biochemical studies indicate that only 25 amino acids are essential for high affinity binding (3). It has high binding affinity to streptavidin because both the N- and C-terminal ends of the 25-amino-acid tag can bind simultaneously to the binding pockets in streptavidin, a tetrameric protein with a binding pocket in each subunit (3). One streptavidin can bind two SBP tags. Since the peptide binding pocket in each streptavidin subunit is the same as the biotin binding pocket in the subunit, biotin binding can effectively displace the bound SBP tag. Natural streptavidin captures biotin extremely tight (Kd ~10-14M). This streptavidin mutein has two mutations to weaken the interactions between biotin and streptavidin (1). This leads to biotin binding in a reversible manner. To simplify the preparation of the affinity matrix, the streptavidin mutein is fused to an agarose binding domain (4). The SAVSBPM18-ABD fusion binds tightly to Sepharose CL-6B. Affinity matrices prepared in this manner show comparable properties as matrices prepared via chemical coupling.
From the laboratory of Sui-Lam Wong, PhD, University of Calgary.
Streptavidin SBPM18 is a streptavidin mutein that can bind both biotinylated proteins and SBP tagged proteins with high affinity (Kd ~10-8M) in a reversible manner.
Highlights:
- Provided as SBPM18 mutein-Sepharose CL-6B affinity matrix
- Ideal for affinity purification of both biotinylated biomolecules and recombinant proteins tagged with streptavidin binding peptide (SBP) tags
- Target proteins can be affinity purified in high purity and with excellent recovery
- Mild conditions are used for elution of target proteins and regeneration of the column
- Many buffers can be used for in the binding, washing and elution steps
- The affinity matrix is reusable
Streptavidin binding peptide (SBP) tag is a peptide tag that binds to streptavidin with nmolar binding affinity (2). Although the original peptide tag is 38 amino acids in length, crystallographic and biochemical studies indicate that only 25 amino acids are essential for high affinity binding (3). It has high binding affinity to streptavidin because both the N- and C-terminal ends of the 25-amino-acid tag can bind simultaneously to the binding pockets in streptavidin, a tetrameric protein with a binding pocket in each subunit (3). One streptavidin can bind two SBP tags. Since the peptide binding pocket in each streptavidin subunit is the same as the biotin binding pocket in the subunit, biotin binding can effectively displace the bound SBP tag. Natural streptavidin captures biotin extremely tight (Kd ~10-14M). This streptavidin mutein has two mutations to weaken the interactions between biotin and streptavidin (1). This leads to biotin binding in a reversible manner. To simplify the preparation of the affinity matrix, the streptavidin mutein is fused to an agarose binding domain (4). The SAVSBPM18-ABD fusion binds tightly to Sepharose CL-6B. Affinity matrices prepared in this manner show comparable properties as matrices prepared via chemical coupling.
From the laboratory of Sui-Lam Wong, PhD, University of Calgary.
| Product Type: | Protein |
| Name: | Streptavidin M18-ABD Sepharose CL-6B |
| Accession ID: | P22629 |
| Format: | Preswollen gel matrix in a 50% suspension. A two ml suspension can generate a column with 1 ml packed bed volume |
| Buffer: | Present in PBS with 0.01% sodium azide |
| Tested Applications: | Affinity chromatography purification of biotinylated proteins and SBP (streptavidin binding tag) tagged proteins |
| Comments: |
Particle Size/Mesh: 45-145 um. Majority is around 50 um in diameter Binding Capacity: 700 ug biotinylated BSA (12 biotin/BSA)/ml matrix Resin Reconstitution: After packing the matrix to the column, rinse the column with TBS (50 mM Tris, 150 mM NaCl pH 7.2). Recommended Mobile Phase: TBS (50 mM Tris, 150 mM NaCl pH 7.2) |
| Storage: | 2-4 degree (C) in TBS |
| Shipped: | Cold Packs |
- Approximately 500 ug of M18-ABD is immobilized per ml of matrix.
- Monomeric MW of M18-ABD is 33841 gm/mole.
- Approximately 15 nmoles of M18-ABD (15 nmoles of biotin binding site) per ml of matrix in our prep.
Very mild conditions (washing with a buffer containing biotin) are sufficient to elute the bound target proteins from the matrices. Extensive wash of the column with the buffer without biotin can effectively regenerate the streptavidin mutein matrix for the next round of purification.
See references 1 and 4 for detailed operating conditions.
- Wu, S. C. & Wong, S. L. Structure-guided design of an engineered streptavidin with reusability to purify streptavidin-binding peptide tagged proteins or biotinylated proteins. PloS one 8, e69530, doi:10.1371/journal.pone.0069530 (2013).
- Keefe, A. D., Wilson, D. S., Seelig, B. & Szostak, J. W. One-step purification of recombinant proteins using a nanomolar-affinity streptavidin-binding peptide, the SBP-Tag. Protein Expr.Purif. 23, 440-446 (2001).
- Barrette-Ng, I. H., Wu, S. C., Tjia, W. M., Wong, S. L. & Ng, K. K. The structure of the SBP-Tag-streptavidin complex reveals a novel helical scaffold bridging binding pockets on separate subunits. Acta crystallographica. Section D, Biological crystallography 69, 879-887, doi:10.1107/S0907444913002576 (2013).
- Wu, S. C., Wang, C., Hansen, D. & Wong, S. L. A simple approach for preparation of affinity matrices: Simultaneous purification and reversible immobilization of a streptavidin mutein to agarose matrix. Sci Rep 7, 42849. doi: 10.1038/srep42849 (2017).
If you publish research with this product, please let us know so we can cite your paper.