SUMO Protease (Ulp1p, M1_K402del)
Yeast SUMO protease variant corresponding to the active SUMO protease domain (amino acids Leu403-Lys621) expressed recombinantly in and purified from E. coli..
Highlights:
- Highly specific and active protease for efficient cleavage of SUMO tags from proteins of interest
- Contains N-terminal polyhistidine tag; Useful for further downstream purification steps
The SUMO domain can act to improve expression and stability of proteins made in E. coli. Hence, SUMO-fusion proteins hold great interest for those performing protein expression in E. coli. The SUMO protease is a highly specific and active protease that can be used to cleave the bond between the SUMO tag and the protein of interest. Further purification, for example by Nickel-affinity columns, can capture the SUMO tag and SUMO protease, yielding a highly pure protein of interest.
From the laboratory of Arnon Lavie, PhD, University of Illinois at Chicago.
Yeast SUMO protease variant corresponding to the active SUMO protease domain (amino acids Leu403-Lys621) expressed recombinantly in and purified from E. coli..
Highlights:
- Highly specific and active protease for efficient cleavage of SUMO tags from proteins of interest
- Contains N-terminal polyhistidine tag; Useful for further downstream purification steps
The SUMO domain can act to improve expression and stability of proteins made in E. coli. Hence, SUMO-fusion proteins hold great interest for those performing protein expression in E. coli. The SUMO protease is a highly specific and active protease that can be used to cleave the bond between the SUMO tag and the protein of interest. Further purification, for example by Nickel-affinity columns, can capture the SUMO tag and SUMO protease, yielding a highly pure protein of interest.
From the laboratory of Arnon Lavie, PhD, University of Illinois at Chicago.
| Catalog Number | Product | DataSheet | Size | AVAILABILITY | Price | Qty |
|---|
This product is for sale to Nonprofit customers only. For profit customers, please Contact Us for more information.
| Product Type: | Protein |
| Name: | Yeast SUMO protease (Ulp1p; protease domain; M1_K402del) |
| Accession ID: | E7KUV8, protease domain |
| Source: | Recombinant expression in E. coli |
| Molecular Weight: | 28,168 Da |
| Amino Acid Sequence: | MGSSHHHHHHSSGGTENLYFQGHMLVPELNEKDDDQVQKALASRENTQLMNRDNIEITVRDFKTLAPRRWLNDTIIEFFMKYIEKSTPNTVAFNSFFYTNLSERGYQGVRRWMKRKKTQIDKLDKIFTPINLNQSHWALGIIDLKKKTIGYVDSLSNGPNAMSFAILTDLQKYVMEESKHTIGEDFDLIHLDCPQQPNGYDCGIYVCMNTLYGSADAPLDFDYKDAIRMRRFIAHLILTDALK |
| Fusion Tag(s): | N-terminal polyhistidine tag |
| Purity: | >95% |
| Buffer: | 12.5 mM Tris (pH 7.5), 250 mM NaCl, 125 mM Imidazole, 1 mM DTT, 50 % glycerol |
| Concentration: | 3.7mg/mL |
| Activity: | Cutting will be dependent on spacing between the SUMO tag and the rest of the protein. Hence, it is hard to define activity. In most cases, 1 hour incubation at a ratio 1:200 at room temperature will result in >99% cleavage of the SUMO tag. |
| Suggested Amount per Experiment: | 10-1000ug, To remove the SUMO tag, incubate the SUMO-X fusion protein with SUMO protease at a ratio of 200:1 (w:w) |
| Shipped: | Dry Ice |
SDS-PAGE Analysis
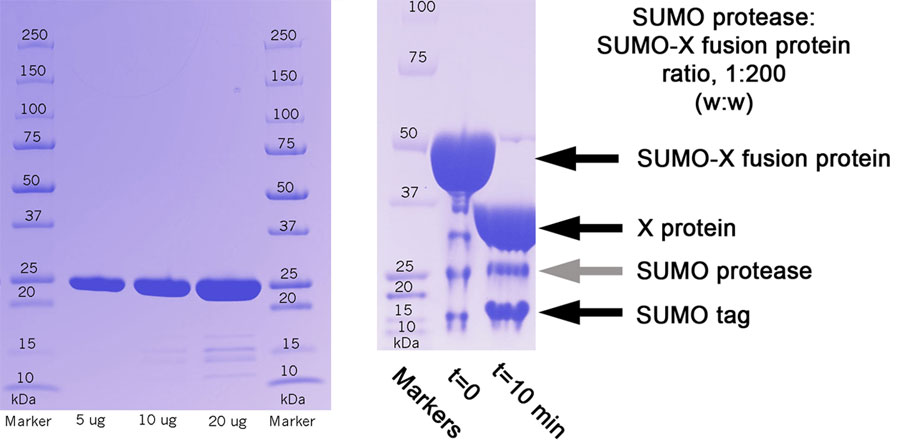
(Left) Purity of protein preperation. (Right) Protein X was expressed as a SUMO fusion protein. To remove the SUMO tag, the SUMO-X fusion protein was incubated with SUMO protease at a ratio of 200:1 (w:w); after 10 minutes, >99% of the protein was cleaved.
1. Kim L, Kwon DH, Heo J, Park MR, Song HK. Use of the LC3B-fusion technique for biochemical and structural studies of proteins involved in the N-degron pathway. J Biol Chem. 2020;295(9):2590-2600. View article
If you publish research with this product, please let us know so we can cite your paper.


