Aqueous Palladium Catalyst
A phosphine-free effective palladium catalyst for aqueous Suzuki-Miyaura Cross-Coupling.
Highlights:
- Ready to use (liquid form), no sample preparation needed
- Allows for the mild coupling between aryl-boronic acids and substrates from small molecules, peptides, proteins and modified cell surface
The Suzuki reaction (sometimes to referred to as the Suzuki-Miyaura reaction or "Suzuki Coupling") is an organic reaction that is classified as a coupling reaction where the coupling partners are a boronic acid with a halide catalyzed by a palladium complex.
From the laboratory of Benjamin G. Davis, PhD, University of Oxford.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
A phosphine-free effective palladium catalyst for aqueous Suzuki-Miyaura Cross-Coupling.
Highlights:
- Ready to use (liquid form), no sample preparation needed
- Allows for the mild coupling between aryl-boronic acids and substrates from small molecules, peptides, proteins and modified cell surface
The Suzuki reaction (sometimes to referred to as the Suzuki-Miyaura reaction or "Suzuki Coupling") is an organic reaction that is classified as a coupling reaction where the coupling partners are a boronic acid with a halide catalyzed by a palladium complex.
From the laboratory of Benjamin G. Davis, PhD, University of Oxford.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
Specifications
| Product Type: | Small Molecule |
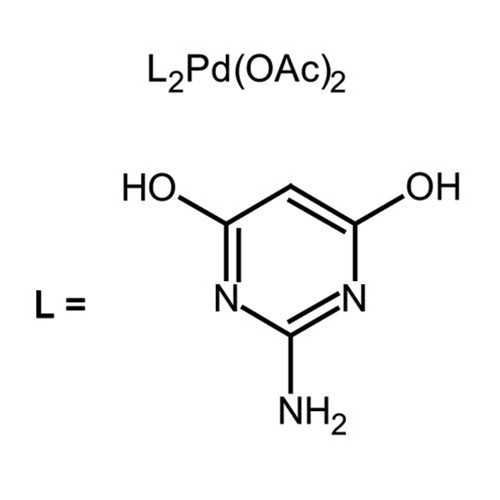
| Name: | Bis(2-amino-4,6-dihydroxypyrimidine)palladium(II) diacetate |
| Chemical Formula: | C12H16N6O8Pd |
| Molecular Weight: | 478.71 kDa |
| Format: | Orange solution |
| Purity: | Pd: 10 mM; transition metal impurity <0.1 mM |
| Solubility: | Soluble in H2O |
| Comments: | 10uL for protein reaction; 2.5mL for 0.25 mol aryl bromide |
| Storage: | Room temperature |
| Shipped: | Ambient temperature |
Provider
From the laboratory of Benjamin G. Davis, PhD, University of Oxford.
References
- Chalker JM, Wood CS, Davis BG. A convenient catalyst for aqueous and protein Suzuki-Miyaura cross-coupling. J. Am. Chem. Soc. 2009, 131, 1634616347.
If you publish research with this product, please let us know so we can cite your paper.