NAPhAz-1
This redox-based probe is ideal for targeting reversible protein tyrosine phosphatase (PTP) oxidation. The probe consists of:
- A “warhead” bearing a cyclic 1,3-diketone group that forms a covalent adduct with the active site cysteine of PTPs
- Naphthalene module that directs binding to the PTP
- Reporter tag used for identification, purification, or direct visualization of the probe-labeled protein.
Protein tyrosine phosphatases (PTPs) are characterized by a catalytic domain that contains an invariant catalytic cysteine with a conserved signature motif (His-Cys-(X)5-Arg-(Ser/Thr)). Owing to its unique environment, the catalytic cysteine has a low pKa (~5.5) and exists predominantly as a thiolate anion at physiological pH, which enhances its nucleophilicity, but also renders it susceptible to oxidation by reactive oxygen species (ROS), which promotes phosphorylation dependent signaling cascades.
From the laboratory of Kate Carroll, PhD, The Scripps Research Institute.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
This redox-based probe is ideal for targeting reversible protein tyrosine phosphatase (PTP) oxidation. The probe consists of:
- A “warhead” bearing a cyclic 1,3-diketone group that forms a covalent adduct with the active site cysteine of PTPs
- Naphthalene module that directs binding to the PTP
- Reporter tag used for identification, purification, or direct visualization of the probe-labeled protein.
Protein tyrosine phosphatases (PTPs) are characterized by a catalytic domain that contains an invariant catalytic cysteine with a conserved signature motif (His-Cys-(X)5-Arg-(Ser/Thr)). Owing to its unique environment, the catalytic cysteine has a low pKa (~5.5) and exists predominantly as a thiolate anion at physiological pH, which enhances its nucleophilicity, but also renders it susceptible to oxidation by reactive oxygen species (ROS), which promotes phosphorylation dependent signaling cascades.
From the laboratory of Kate Carroll, PhD, The Scripps Research Institute.
 Part of The Investigator's Annexe program.
Part of The Investigator's Annexe program.
| Catalog Number | Product | DataSheet | Size | AVAILABILITY | Price | Qty |
|---|
| Product Type: | Small Molecule |
| Name: | NAPhAz-1 |
| Chemical Formula: | C21H21N5O4 |
| Molecular Weight: | 407.1594 g/mol |
| Format: | Solid |
| Purity: | >95%, HPLC |
| Solubility: | DMSO |
| Stability: | >6 months at -20C |
| Spectral Information: | 1H NMR (400 MHz, DMSO-d6): δ 8.29 (s, 2H), 7.92 (d, J=8.8, 1H),7.84 (s, 2H), 7.63 (d, J=8.8, 1H), 5.10 (s, 1H), 3.49 (t, J=4.4, 2H), 3.43 (t, J=6.8, 2H), 2.75-2.53 (m, 4H), 1.9 (q, J=7.2, 2H), 1.56 (t, J=7.2, 1H) |
| Storage: | Upon receipt, prepare a 250 mM stock solution in DMSO and store at -20C. |
| Shipped: | Dry ice |
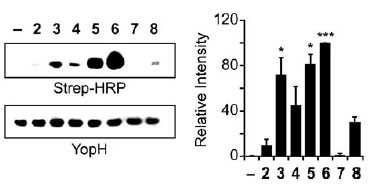
Analysis of BiPhAz-1 and NAPhAz-1 selectivity for oxidized PTP YopH. BiPhAz-1(compound 6) and NAPhAz-1 (compound 5) detect sulfenic acid in oxidized YopH with increased sensitivity over the parent compound DAz-1. YopH was oxidized with H2O2 and incubated with compounds 28 or DMSO alone (). Left: Following p-biotin conjugation, reactions were analyzed by streptavidin-HRP western blot. Right: Equal loading was verified by Ponceau S staining.
Adapted from: Leonard, S. E., Garcia, F. J., Goodsell, D. S., and Carroll, K. S. (2011) Redox-based probes for protein tyrosine phosphatases, Angew Chem Int Ed Engl 50, 4423-4427.
- Leonard, S. E., Garcia, F. J., Goodsell, D. S., and Carroll, K. S. (2011) Redox-based probes for protein tyrosine phosphatases, Angew Chem Int Ed Engl 50, 4423-4427.
- Truong, T. H., and Carroll, K.S. (2012) Bioorthogonal Chemical Reporters for Analyzing Protein Sulfenylation in Cells, Curr Protoc Chem Biol 4, 101-122.
If you publish research with this product, please let us know so we can cite your paper.


